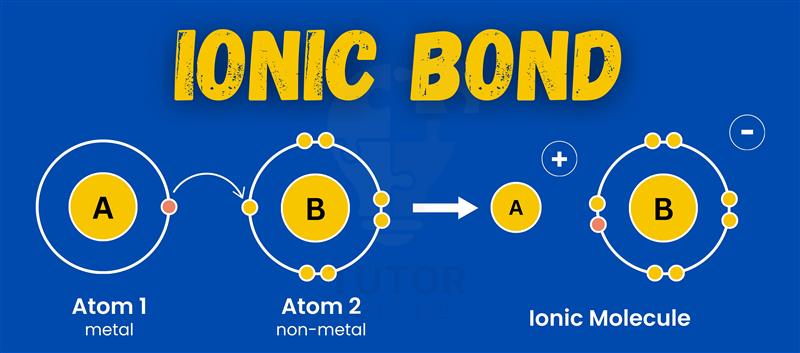
Have you ever thought about why table salt is so stable? The answer lies in ionic bonds.
These bonds form when one atom gives electrons to another. It’s like a chemical exchange! The resulting oppositely charged ions attract, creating strong bonds.
In this guide, you will learn What Is an Ionic Bond? types, formation, and properties of ionic bonds.
What Is an Ionic Bond?
An ionic bond forms when one atom transfers electrons to another, creating oppositely charged ions that attract each other.
Examples of Ionic Bonds:
- Sodium Chloride (NaCl): Sodium donates one electron to chlorine, forming Na⁺ and Cl⁻ ions.
- Magnesium Oxide (MgO): Magnesium transfers two electrons to oxygen, resulting in Mg²⁺ and O²⁻ ions.
Types of Ionic Bonds
Ionic bonds come in two main types, depending on the nature of the cation involved. Let me explain them for you!
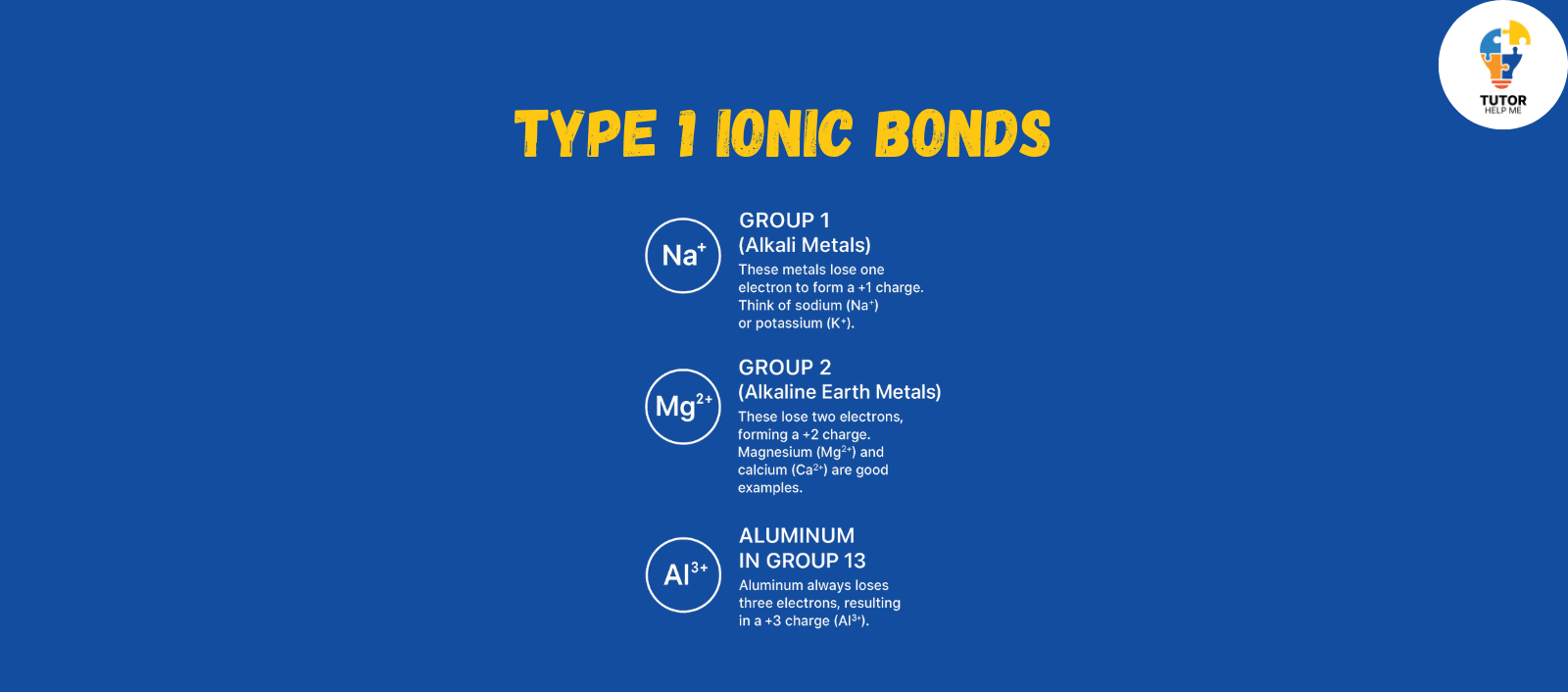
Type 1 Ionic Bonds
Type 1 ionic bonds are the simplest. In type 1 Ionic bonds the cation always has the same charge, making it predictable and easy to identify. These cations typically come from:
- Group 1 (Alkali Metals): These metals lose one electron to form a +1 charge. Think of sodium (Na⁺) or potassium (K⁺).
- Group 2 (Alkaline Earth Metals): These lose two electrons, forming a +2 charge. Magnesium (Mg²⁺) and calcium (Ca²⁺) are good examples.
- Aluminum in Group 13: Aluminum always loses three electrons, resulting in a +3 charge (Al³⁺).
These bonds are straightforward because the cation’s charge doesn’t change. It’s reliable, making Type 1 ionic bonds easy to work with.
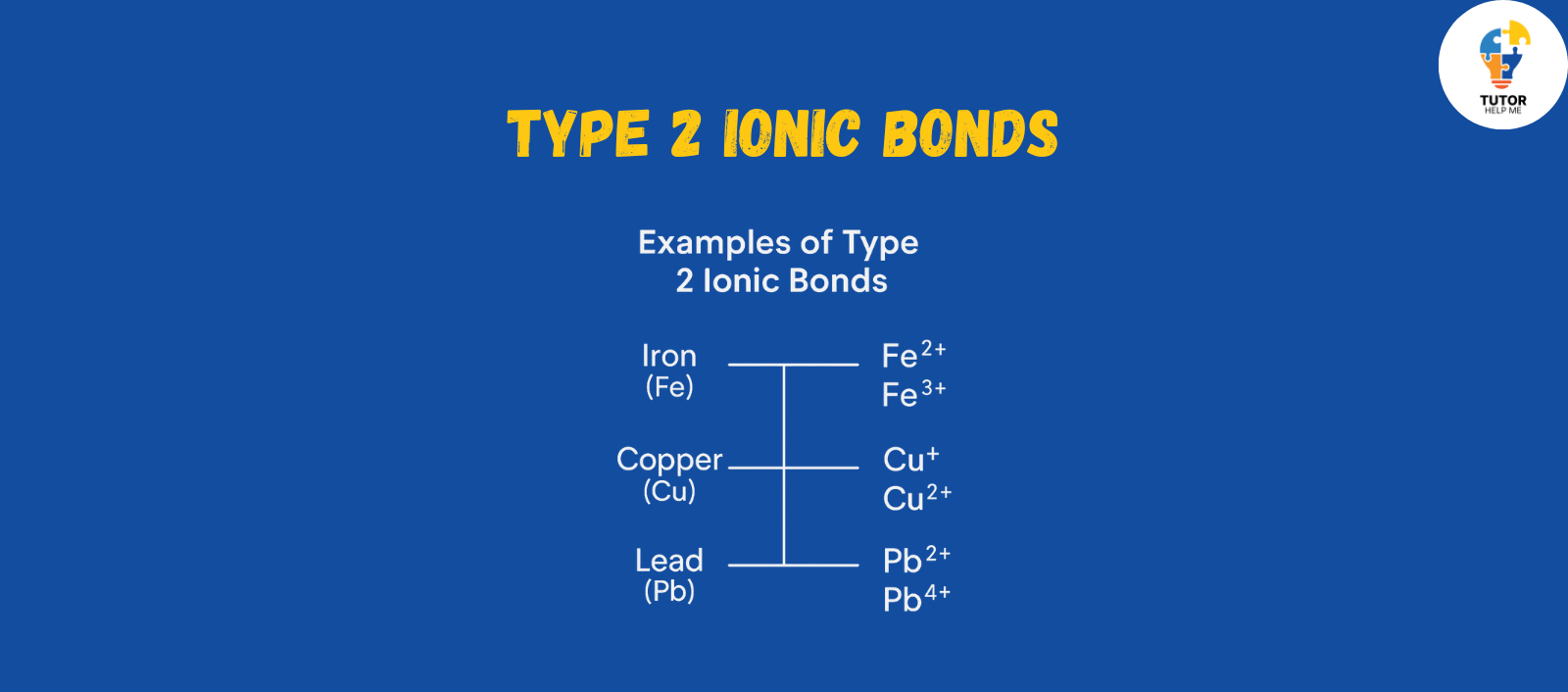
Type 2 Ionic Bonds
Type 2 ionic bonds are a bit more exciting. In type 2 Ionic bonds the cation can have multiple charges depending on the situation. This makes them less predictable but adds variety. These cations usually come from transition metals, such as:
- Iron (Fe): It can lose different numbers of electrons to form Fe²⁺ or Fe³⁺.
- Copper (Cu): Copper can form either Cu⁺ or Cu²⁺ ions.
- Lead (Pb): Lead shows its versatility by forming Pb²⁺ or Pb⁴⁺ ions.
Since these metals can form different charges, you will see Roman numerals in their chemical formulas.
For example:
- FeCl₂ is iron(II) chloride (Fe²⁺).
- FeCl₃ is iron(III) chloride (Fe³⁺).
How Are Ionic Bonds Formed?
Ionic bonds are formed through a simple process:
1. Electron Transfer:
The first thing that happens is that a metal atom gives away one or more of its outer electrons. This metal atom now becomes a positively charged ion (called a cation). On the other hand, a non-metal atom takes in these electrons and becomes a negatively charged ion (called an anion).
2. Attraction Between Opposite Charges:
Once the metal loses its electrons and the non-metal gains them, the two ions now have opposite charges. And we know that opposites attract! The positive cation and negative anion pull towards each other because of this strong attraction.
3. Lattice Formation:
Now, these ions don’t just stay as isolated pairs. They arrange themselves in a repeating three-dimensional pattern known as a crystal lattice. This pattern is very stable and gives ionic compounds their unique properties, like high melting and boiling points.
For example, in sodium chloride (NaCl):
- Sodium (Na) loses one electron to become Na⁺.
- Chlorine (Cl) gains that electron to become Cl⁻.
The Na⁺ and Cl⁻ ions attract each other, forming a strong ionic bond that creates salt!
Isn’t it cool how this simple process builds something so useful?
Properties of Ionic Bonds
Here are the key properties of ionic bonds with examples to make it clearer:
- High Melting and Boiling Points:
Ionic compounds need a lot of heat to melt or boil because their bonds are strong. For example, sodium chloride (NaCl), or table salt, has a high melting point and doesn’t melt easily. - Hard and Brittle:
Ionic compounds are hard because their ions are tightly packed. But, if you hit them, they can break or shatter. For example, calcium carbonate (CaCO₃) is hard but can break easily if hit. - Conduct Electricity (When Melted or Dissolved):
Ionic compounds don’t conduct electricity when solid, but when melted or dissolved in water, their ions can move and carry electricity. For example, sodium chloride (NaCl) can conduct electricity when dissolved in water. - Dissolve in Water:
Many ionic compounds dissolve easily in water. The water pulls the ions apart, making them mix with the liquid. For instance, potassium chloride (KCl) dissolves easily in water. - Strong Bonds:
The attraction between positive and negative ions is very strong, making ionic bonds stable and reliable. Magnesium oxide (MgO) is a good example of a compound with very strong ionic bonds. - Crystal Lattice Structure:
Ionic compounds form a regular pattern of ions, which makes them strong and gives them their special shape. Sodium chloride (NaCl) forms a cubic crystal structure, showing how ions are arranged neatly.
These properties help explain why ionic compounds are used in so many everyday items!
Why Do Ionic Compounds Conduct Electricity in Water?
Ionic compounds do not conduct electricity in their solid state because the ions are fixed in place. However, when dissolved in water, the ions separate and move freely, allowing electric current to pass through.
This is why electrolytes are essential in batteries and biological functions.
How Does Ionic Bonding Affect Material Strength?
Materials with strong ionic bonds are often:
- Used in construction (e.g., calcium carbonate in cement).
- Important for insulation (e.g., magnesium oxide in heat-resistant materials).
- Key in electronics (e.g., lithium salts in rechargeable batteries).
Understanding ionic bonding helps in choosing durable and efficient materials for various applications.
Why Choose TutorHelpMe Chemistry tutors?
- Expert Guidance: Learn from qualified Chemistry tutors with deep knowledge of chemistry.
- Personalized Lessons: Tailored sessions to suit your learning needs.
- Improved Grades: Boost performance with clear concepts and effective strategies.
- Interactive Sessions: Engaging lessons for better understanding.
- Exam Preparation: Focused support for exams and assignments.
- Affordable Rates: Quality tutoring that fits your budget.
- Flexible Options: Learn from home with online sessions.
- All Levels Covered: Support for GCSE, A-Level, and more.
- Friendly Tutors: Patient and approachable, making learning enjoyable.
- Proven Results: Trusted by students for academic success.
Read More What is covalent bond? Properties and Types Of Covalent Bond
FAQ’s
What are Cation?
A cation is a positively charged ion formed when an atom loses one or more electrons.
what is lattice formation?
Lattice formation is the arrangement of atoms, ions, or molecules in a repeating, orderly pattern in a solid.
How Are Ionic Compounds Used in Medicine?
Many pharmaceutical companies use ionic compounds in medications and supplements. For example, sodium chloride (NaCl) is essential for IV fluids, and calcium ions help in bone-strengthening treatments.