Have you ever felt the warmth of the sun or the heat from a cup of tea? That warmth is heat energy, one of the most important forms of energy in our daily lives.
Heat energy is responsible for cooking food, keeping us warm, and powering machines. In science, understanding what heat energy is helps explain how energy moves and changes from one object to another.
In this detailed guide, we’ll explore what is heat energy, its formula, key aspects, types, and ways it transfers all in simple words and real-life examples.
What Is Heat Energy?
Heat energy is the energy transferred between objects or systems due to a difference in temperature. It always moves from something hotter to something colder until both reach the same temperature.
Example:
When you place a metal spoon in hot tea, the spoon becomes warm because heat moves from the tea (hot) to the spoon (cold).
Heat Energy Formula and Equations
The amount of heat energy can be calculated using this formula:
Q = m × c × ΔT
Where:
- Q = Heat energy (in joules, J)
- m = Mass of the substance (in kilograms, kg)
- c = Specific heat capacity (in J/kg°C)
- ΔT = Change in temperature (in °C)
Example:
If you heat 2 kg of water by 10°C and the specific heat capacity of water is 4184 J/kg°C,
then:
Q= 2 × 4184 × 10 = 83,680 J
So, 83,680 joules of heat energy are required.

Key Aspects of Heat Energy
Let’s understand what makes heat energy so special by breaking it into key aspects:
a) Molecular Motion
Heat energy comes from the movement of particles. When particles move faster, the object becomes hotter.
- Example: When you heat water, the water molecules start moving rapidly, increasing its temperature.
b) Temperature vs. Heat
People often confuse temperature with heat, but they are not the same.
- Temperature measures how hot or cold something is.
- Heat measures the energy transferred due to that temperature difference.
Example:
A spark may have a higher temperature than boiling water, but it has less heat energy because it has less mass.
c) Energy Transfer
Heat energy always moves from a hotter object to a cooler one.
- Example: When you touch an ice cube, heat moves from your hand to the ice, making it melt.
d) Phase Changes
Heat energy can change the state of matter, solid, liquid, or gas, without changing the temperature.
- Example:
- Ice melting into water absorbs heat.
- Water boiling into steam also absorbs heat.
This type of heat is called latent heat.
e) Measurement
Heat energy is measured in:
- Joules (J) — the standard unit
- Calories (cal) — commonly used in food energy
- British Thermal Units (BTU) — often used in heating systems
Conversion:
1 calorie = 4.184 joules
Ways of Transferring Heat Energy
There are three main ways heat energy transfers from one object to another: conduction, convection, and radiation.
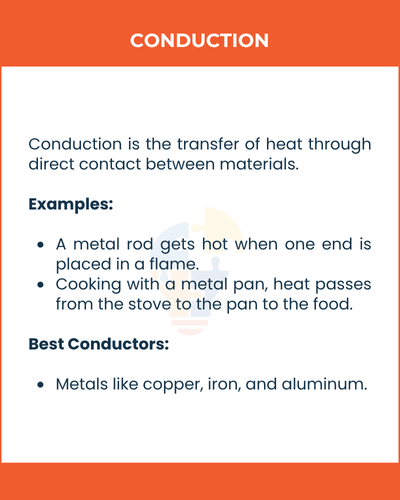
1) Conduction
Conduction is the transfer of heat through direct contact between materials.
How it works:
When two objects touch, the faster-moving particles of the hotter object collide with the slower ones of the cooler object, transferring energy.
Examples:
- A metal rod gets hot when one end is placed in a flame.
- Cooking with a metal pan, heat passes from the stove to the pan to the food.
Best Conductors:
- Metals like copper, iron, and aluminum.
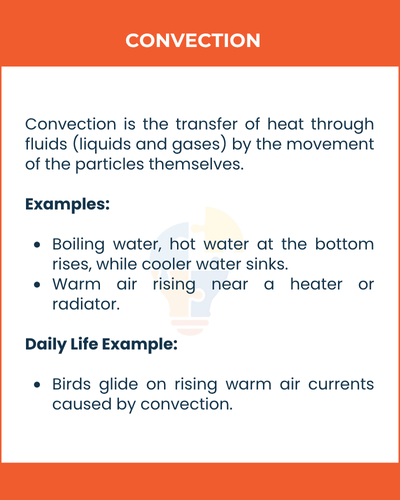
2) Convection
Convection is the transfer of heat through fluids (liquids and gases) by the movement of the particles themselves.
How it works:
Hot fluids rise, and cool fluids sink, creating a convection current that transfers heat.
Examples:
- Boiling water, hot water at the bottom rises, while cooler water sinks.
- Warm air rising near a heater or radiator.
Daily Life Example:
Birds glide on rising warm air currents caused by convection.
3) Radiation
Radiation is the transfer of heat through electromagnetic waves, without any physical contact.
How it works:
Energy travels in waves and can move through empty space (a vacuum).
Examples:
- Feeling warmth from sunlight.
- Heat from a campfire reaching your face even from a distance.
Good Absorbers and Emitters:
Dark-colored objects absorb and radiate more heat than light-colored ones.

Types of Heat Energy
Heat energy can be categorized based on how it’s used or transferred:
1) Sensible Heat
This is the heat that causes a change in temperature of a substance.
- Example: Heating water from 25°C to 60°C.
2) Latent Heat
This is the heat that causes a change in the state of matter without changing temperature.
- Example: Ice melting into water at 0°C.
There are two types of latent heat:
- Latent Heat of Fusion: Heat absorbed during melting or released during freezing.
- Latent Heat of Vaporization: Heat absorbed during boiling or released during condensation.
3) Specific Heat Energy
This is the amount of heat required to raise the temperature of 1 kg of a substance by 1°C.
- Example: Water has a high specific heat, meaning it takes more energy to heat it than metal.
Heat Energy in Everyday Life
We experience heat energy in many ways around us:
| Activity | How Heat Energy Works |
|---|---|
| Cooking | Heat transfers from stove to pan to food. |
| Ironing clothes | Heat from the iron removes moisture. |
| Greenhouse effect | Sun’s radiation warms the Earth. |
| Engine working | Fuel combustion produces heat to move pistons. |
| Heating water | Electrical energy converts to heat energy. |
Role of Heat Energy in Nature
- Weather and Climate: Heat energy drives air currents and ocean waves.
- Photosynthesis: The sun’s heat helps plants grow.
- Volcanic Activity: Heat energy from Earth’s core creates lava.
- Water Cycle: Heat from the sun causes evaporation and rainfall.
Fun Facts About Heat Energy
- The Sun’s surface temperature is about 5,500°C!
- Metals expand when heated and contract when cooled.
- Heat travels faster in liquids and solids than in gases.
- Space feels cold because there are no molecules to transfer heat.
Real-Life Example of Heat Transfer
Let’s take a cup of hot tea:
- Conduction: Heat transfers from the tea to the cup.
- Convection: Warm tea rises, and cooler tea sinks.
- Radiation: You feel the heat radiating from the surface.
Importance of Understanding Heat Energy
Understanding what is heat energy helps in many fields:
- Engineering: Designing engines, heaters, and refrigerators.
- Medicine: Managing body temperature.
- Climate Science: Studying global warming.
- Everyday Life: Cooking, heating, and cooling systems.
Conclusion
Heat energy is one of the most essential forms of energy around us. It moves naturally from hot to cold, powering countless processes, from boiling water to driving weather patterns.
By understanding what is heat energy, its transfer methods, and types, we gain a deeper insight into how energy shapes our lives.
From the warmth of sunlight to the heat in engines, this energy keeps the world active, comfortable, and full of life.
Read More What is Wave Energy?
FAQs
Q1: What is heat energy in simple words?
A: Heat energy is energy that moves from a hot object to a cold one.
Q2: What is the formula for heat energy?
A: Q = m × c × ΔT, where Q is heat energy, m is mass, c is specific heat, and ΔT is change in temperature.
Q3: What are the three ways heat energy is transferred?
A: Conduction, convection, and radiation.
Q4: What is an example of conduction?
A: A metal spoon getting hot when placed in hot soup.
Q5: How is heat energy measured?
A: In joules (J), calories (cal), or BTUs.
Q6: What is the difference between heat and temperature?
A: Heat is energy transfer; temperature measures how hot or cold something is.
Q7: What is latent heat?
A: Heat that changes a substance’s state, like ice melting, without changing its temperature.
Q8: What are good conductors of heat?
A: Metals like copper and iron.
Q9: Can heat energy travel through a vacuum?
A: Yes, by radiation, such as sunlight reaching Earth.
Q10: What is convection in daily life?
A: Warm air rising from a heater in a room.
Q11: Why does metal feel cold?
A: It quickly absorbs heat from your skin due to good conductivity.
Q12: What is specific heat?
A: The amount of heat needed to raise the temperature of 1 kg of a substance by 1°C.
Q13: Which type of heat changes matter’s state?
A: Latent heat.
Q14: What are examples of radiation?
A: Sunlight, infrared heaters, and microwaves.
Q15: Does heat energy always flow upward?
A: No, it flows from hot to cold, regardless of direction.
Q16: What are applications of heat energy?
A: Cooking, drying clothes, heating systems, and industrial processes.
Q17: Why is heat energy important in weather?
A: It drives wind, rain, and temperature changes.
Q18: Can we store heat energy?
A: Yes, in water tanks, molten salts, and thermal batteries.
Q19: What are the types of heat energy?
A: Sensible heat, latent heat, and specific heat.
Q20: What is an example of heat energy transfer in nature?
A: Sun warming the Earth through radiation.