Want to know how force spreads over an area? Let’s talk about pressure and density.
Pressure is around you all the time in the air, in water, and even in your body. Understanding it helps explain weather, tools, and everyday things.
In this guide, you will learn what density means, how to find it using a simple formula, and the types of density you may come across.
Ready to learn something new? Sit back, have a cup of tea, Let’s get started!
What is Density?
"Density measures how much mass is packed into a given volume of a substance."
SI Unit of Density,
Kilograms per cubic metre (kg/m³).
Formula of Density,
Density (ρ) = Mass (m) ÷ Volume (V)
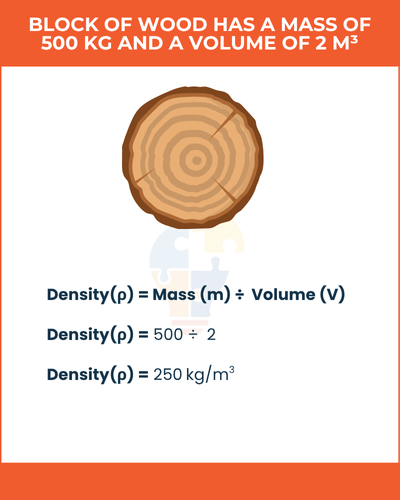
Example of Density,
If a block of wood has a mass of 500 kg and a volume of 2 m³, its density is:
Density(ρ) = Mass (m) ÷ Volume (V) = 500 ÷ 2 = 250 kg/m3
This means the wood’s density is 250 kg/m³.
Why is Density Important in Everyday Life?
Density affects everything around us. It determines whether an object floats or sinks, why hot air balloons rise, and even how metals are chosen for construction. Understanding density helps in various fields, including engineering, physics, and chemistry.
Types of Density:
- Absolute density
- Relative density
- Bulk density
- Particle density
- Neutral density
- Area density
- Linear density
- Vapor density
- Agricultural density
Absolute density
"Absolute density refers to the mass of a substance per unit volume without considering external factors like temperature or pressure."
SI Unit of Absolute density,
Kilograms per cubic metre (kg/m³).
Formula of Absolute density,
Absolute Density (ρ) = Mass (m) ÷ Volume (V)
Example of Absolute Density,
If a metal block has a mass of 800 kg and a volume of 0.4 m³, its absolute density is:
Absolute Density (ρ)=Mass (m) ÷ Volume (V) = 800 ÷ 0.4 = 2000 kg/m3
This means the metal’s absolute density is 2000 kg/m³.

Relative Density:
"Relative density compares the density of a substance to the density of water."
SI Unit of Relative Density,
It has no unit since it is a ratio.
Formula of Relative Density,
Relative Density = Density of substance ÷ Density of water
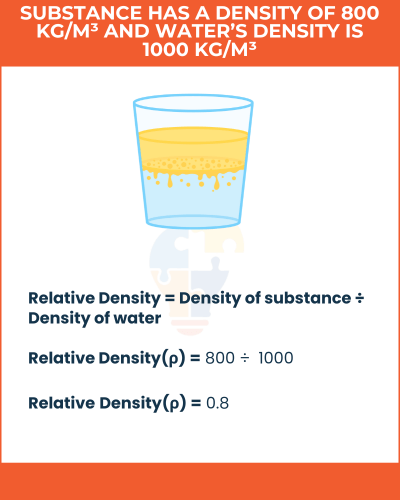
Example of Relative Density,
If a substance has a density of 800 kg/m³ and water’s density is 1000 kg/m³, its relative density is:
800 ÷ 1000 = 0.8
Bulk Density
"Bulk density is the mass of a substance per unit volume, including air space."
SI Unit of Bulk Density,
Si unit is kg/m³.
Formula of Bulk Density,
Bulk Density = Mass ÷ Volume (including voids)
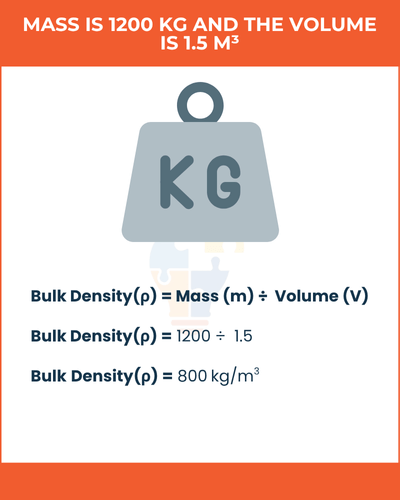
Example of Bulk Density,
If the mass is 1200 kg and the volume is 1.5 m³, bulk density is:
1200/1.5 = 800kg/m3
Particle Density
"Particle density measures the mass per unit volume of individual particles."
SI Unit of Particle Density,
Si unit is kg/m³.
Formula of Particle Density,
Particle Density = Mass of particles ÷ Volume of particles
Example of Particle Density,
A particle with mass 300 kg and volume 0.1 m³ has particle density 300 ÷ 0.1 = 3000 kg/m3
Neutral Density
"Neutral density compares the density of a substance to water, considering equal temperature and pressure."
SI Unit of Neutral Density,
It has no unit since it is a ratio.
Formula of Neutral Density,
Neutral Density = Density of substance ÷ Density of water at standard conditions
Example of Particle Density,
A particle with mass 300 kg and volume 0.1 m³ has particle density 300 ÷ 0.1 = 3000 kg/m3.
Area Density
"Area density measures the mass per unit area of a surface."
SI Unit of Area Density,
Si unit is kg/m².
Formula of Area Density,
Area Density = Mass ÷ Area
Example of Area Density,
A material with mass 50 kg and area 2 m² has area density 50 ÷ 2 = 25 kg/m2.
Linear Density
"Linear density measures mass per unit length."
SI Unit of Linear Density,
Si unit is kg/m.
Formula of Linear Density,
Linear Density = Mass ÷ Length
Example of Linear Density,
A wire with mass 200 g and length 5 m has linear density 200 ÷ 5=40 g/m.
Vapor Density
"Vapor density compares the density of a gas to the density of hydrogen at the same temperature and pressure."
SI Unit of Vapour Density,
It has no unit since it is a ratio.
Formula of Vapour Density,
Vapor Density = Density of gas ÷ Density of hydrogen
Example of Vapour Density,
A gas with density 2.2 kg/m³ and hydrogen’s density 0.09 kg/m³ has vapor density 2.2 ÷ 0.09 = 24.44 .
How Does Temperature Affect Density?
- Solids and liquids: Density usually decreases as temperature increases because molecules move apart.
- Gases: Warmer air is less dense than cooler air, which is why hot air rises.
This principle is essential in weather predictions, refrigeration, and material science.
What is the Relationship Between Density and Buoyancy?
Buoyancy depends on density. Objects denser than water sink, while less dense objects float. This principle is key in designing ships, submarines, and aircraft.
Why Choose TutorHelpMe Physics Tutors?
- Qualified Experts: Physics Tutors with in-depth knowledge of physics and years of teaching experience.
- Tailored Lessons: Customised sessions based on your learning pace and academic needs.
- Exam-Focused Preparation: Strategies and resources to excel in exams and tests.
- Concept Clarity: Simplified explanations to make complex topics easy to understand.
- Interactive Learning: Engaging teaching methods to keep you motivated and focused.
- Flexible Scheduling: Study at times that suit your availability and routine.
- Affordable Pricing: High-quality tutoring at a budget-friendly cost.
- Practical Applications: Relating physics concepts to real-world examples for better understanding.
- Doubt Clearance: One-on-one sessions to address all your questions and challenges.
- Global Access: Online tutoring available from anywhere, ensuring convenience and accessibility.
- Proven Results: Students achieve improved grades and enhanced confidence in physics.
- Progress Tracking: Regular feedback and assessments to monitor growth.
Choose TutorHelpMe for a better physics learning experience!
Read More What is Velocity? Units, Formula, Types
FAQ’s
What is the easiest way to understand density?
Density is simply how much mass is packed into a certain volume. Imagine holding a brick and a sponge of the same size—the brick feels heavier because it has a higher density.
What are the most common units of density?
The standard unit is kilograms per cubic metre (kg/m³). However, grams per cubic centimetre (g/cm³) is also used in laboratories and schools.
How does density affect floating and sinking?
An object will float if it is less dense than the liquid it’s placed in. For example, boats float on water despite being made of metal, as their overall density is lower due to air-filled spaces.
Why does ice float on water?
Ice is less dense than liquid water, which is unusual because most substances get denser when they freeze. This property is why lakes freeze from the top down, protecting aquatic life.
Can density change?
Yes! Temperature and pressure affect density. Gases, for example, expand when heated, becoming less dense. This principle is why hot air balloons rise.
How does density affect the weather?
Air density impacts air pressure and wind patterns. Warm air rises, creating low-pressure systems, which lead to cloud formation and rain—important in UK weather forecasting!
What is the densest natural substance?
Osmium, a rare metal, has the highest density of any naturally occurring element, making it useful in medical and industrial applications.