Acids, bases, and salts are essential substances in chemistry, influencing many aspects of day-to-day life. From the sour taste of citrus fruits like lemons and oranges to the bitter taste of household cleaners, these compounds play a key role in science, industry, and even cooking.
Acids and bases are widely used in laboratories and industries. For example, hydrochloric acid (HCl) is used in gastric processes and industrial cleaning, while sodium hydroxide (NaOH) is essential in soap production. Salts, such as sodium chloride (NaCl), are found in everyday cooking and chemical manufacturing.
This guide will explore:
- What Are Acids, Bases, and Salts?
- The properties of acids and bases.
- The pH scale and how to test for acidity and alkalinity.
- The neutralisation process and the formation of salts.
- Common uses of acids, bases, and salts in daily life.
Understanding these substances not only helps in GCSE chemistry but also explains their importance in medicine, agriculture, and industrial applications.
What Are Acids, Bases, and Salts?
Acids, bases, and salts are essential substances in chemistry, found in nature, household products, and industries. These substances play a significant role in chemical reactions, cooking, medicine, and environmental processes.
Acids
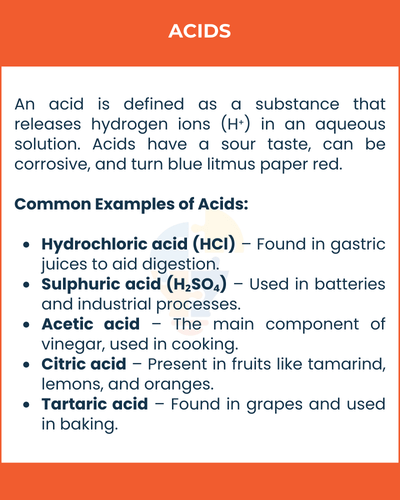
An acid is defined as a substance that releases hydrogen ions (H⁺) in an aqueous solution. Acids have a sour taste, can be corrosive, and turn blue litmus paper red.
Common Examples of Acids
- Hydrochloric acid (HCl) – Found in gastric juices to aid digestion.
- Sulphuric acid (H₂SO₄) – Used in batteries and industrial processes.
- Acetic acid – The main component of vinegar, used in cooking.
- Citric acid – Present in fruits like tamarind, lemons, and oranges.
- Tartaric acid – Found in grapes and used in baking.
Some acids, like acetic acid and tartaric acid, are considered natural acids because they are found in food and plants.
Bases
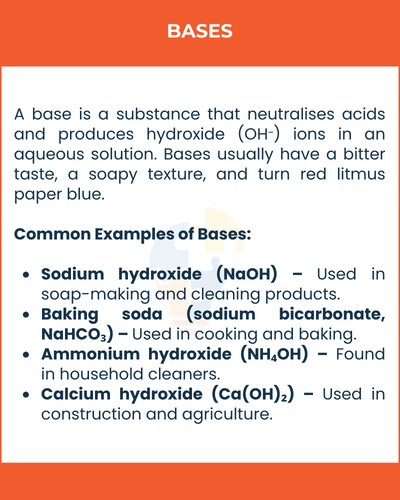
A base is a substance that neutralises acids and produces hydroxide (OH⁻) ions in an aqueous solution. Bases usually have a bitter taste, a soapy texture, and turn red litmus paper blue.
Common Examples of Bases
- Sodium hydroxide (NaOH) – Used in soap-making and cleaning products.
- Baking soda (sodium bicarbonate, NaHCO₃) – Used in cooking and baking.
- Ammonium hydroxide (NH₄OH) – Found in household cleaners.
- Calcium hydroxide (Ca(OH)₂) – Used in construction and agriculture.
Some weak bases are commonly found in day-to-day life, such as antacids, which help relieve acidity in the stomach.
Salts
A salt is an ionic compound formed when an acid and a base react in a neutralisation reaction. Salts are composed of positively charged ions (cations) and negatively charged ions (anions).
Common Examples of Salts
- Sodium chloride (NaCl) – Common table salt, used in cooking.
- Ammonium chloride (NH₄Cl) – Found in batteries and fertilisers.
- Calcium carbonate (CaCO₃) – Used in chalk, limestone, and antacids.
Salts can be classified based on their properties:
- Acid salts – Formed when a strong acid reacts with a weak base.
- Basic salts – Formed when a strong base reacts with a weak acid.

What Is the Difference Between Acids and Bases?
Acids and bases are two fundamental types of chemical substances that behave differently in reactions. Their properties determine how they interact with other chemicals, metals, and water. Understanding these differences is essential in GCSE Chemistry and has real-world applications in science, medicine, and industry.
Properties of Acids vs Bases
The table below highlights the key differences between acids and bases based on their properties:
| Aspect | Acids | Bases |
|---|---|---|
| Definition | Acids are substances that release hydrogen ions (H⁺) in an aqueous solution. | Bases are substances that release hydroxide ions (OH⁻) in an aqueous solution. |
| pH Range | Less than 7 on the pH scale. | Greater than 7 on the pH scale. |
| Taste | Have a sour taste (e.g., citric acid in fruits like lemons and oranges). | Have a bitter taste (e.g., baking soda and sodium hydroxide). |
| Feel | Can be corrosive and sting on contact. | Feel slippery or soapy (e.g., common household bases like soap). |
| Reaction with Metals | Acids react with metals to produce hydrogen gas (e.g., hydrochloric acid with zinc). | Bases do not react with most metals under normal conditions. |
| Reaction with Litmus Paper | Turns blue litmus red. | Turns red litmus blue. |
| Common Examples | Hydrochloric acid (HCl), sulphuric acid (H₂SO₄), acetic acid (vinegar), tartaric acid. | Sodium hydroxide (NaOH), ammonia (NH₃), calcium hydroxide (Ca(OH)₂), baking soda (NaHCO₃). |
| Found in | Gastric juices, vinegar, citrus fruits, acid rain, batteries. | Soap, cleaning products, antacids, laboratories and industries. |
What Happens When Acids and Bases React? (Neutralisation Reaction)
A neutralisation reaction occurs when an acid and a base react to form a salt and water. This reaction is widely used in medicine, agriculture, and industrial processes.
Neutralisation Reaction Equation:
Acid + Base → Salt + Water
For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), the products are sodium chloride (NaCl) (table salt) and water (H₂O):
HCl +NaOH → NaCl + H₂O
Examples of Neutralisation in Everyday Life:
✔ Medicine: Antacids neutralise excess stomach acidity to relieve indigestion.
✔ Agriculture: Farmers use lime (calcium hydroxide) to neutralise acidic soil.
✔ Water Treatment: Neutralisation helps balance pH in drinking water.
How Are Acids and Bases Measured? (pH Scale Explained)
Acids and bases are measured using the pH scale, which helps determine whether a substance is acidic, neutral, or alkaline. Understanding this scale is essential in GCSE Chemistry, laboratories, and day-to-day life.
What Is the pH Scale?
The pH scale ranges from 0 to 14 and measures the acidity or alkalinity of a solution. It works by assessing the concentrationofhydrogen ions (H⁺) and hydroxide ions (OH⁻) in an aqueous solution.
| pH Value | Nature of Solution | Example |
|---|---|---|
| 0 – 6 | Acidic | Hydrochloric acid (HCl), vinegar, gastric juices |
| 7 | Neutral | Pure water, sodium chloride solution |
| 8 – 14 | Alkaline (Basic) | Sodium hydroxide, baking soda, soap |
- A strong acid, such as sulphuric acid, has a low pH (0–3).
- A weak acid, like acetic acid, has a pH of 4–6.
- A strong base, such as sodium hydroxide, has a high pH (12–14).
- A weak base, like baking soda, has a pH of 8–9.
How Do Litmus Paper and Universal Indicators Work?
To measure the pH of a substance, chemists use litmus paper, universal indicators, and digital pH meters.
Litmus Paper Test
- Red litmus paper turns blue when exposed to a base.
- Blue litmus paper turns red when exposed to an acid.
This simple test quickly identifies whether a solution is acidic or alkaline, but it does not provide an exact pH value.
Universal Indicator
Universal indicators give a more precise measurement by changing colour depending on the pH of the solution.
| pH Range | Indicator Colour | Nature of Solution |
|---|---|---|
| 0 – 3 | Red | Strong acid |
| 4 – 6 | Orange/Yellow | Weak acid |
| 7 | Green | Neutral |
| 8 – 10 | Blue | Weak base |
| 11 – 14 | Purple | Strong base |
This method is widely used in schools, laboratories, and industries to test household, industrial, and natural solutions.
"Get expert tutoring from 1-on-1 online GCSE chemistry tutors."
Why Is the pH Scale Important?
The pH scale is essential in various fields:
- In Chemistry: It helps classify acids, bases, and salts.
- In Medicine: Gastric juices in the stomach contain hydrochloric acid, which aids digestion. Antacids, which are weak bases, neutralise excess acidity.
- In Agriculture: Farmers test soil pH to determine whether to add lime (a base) or fertilisers (acidic compounds).
- In Food Industry: Tartaric acid and citric acid are used for preservation and flavour enhancement.
What Are the Common Uses of Acids and Bases?
Acids and bases are essential in day-to-day life, from household cleaning to food preparation and industrial processes. Their properties make them useful in various fields, including medicine, agriculture, and manufacturing.
Uses of Acids
Acids are substances that dissociate in their aqueous solution to form their constituent ions, making them highly reactive and useful in many applications. Some of the most common uses include:
1. Cleaning Products
Many strong acids, such as sulphuric acid and hydrochloric acid (HCl), are used in cleaning solutions.
- Hydrochloric acid is found in toilet cleaners to remove tough stains.
- Acetic acid (found in vinegar) helps clean surfaces naturally.
- Citric acid from fruits like lemons and oranges is used in descaling kettles and removing rust.
These acids react with negatively charged ions in stains, breaking them down effectively.
2. Food Industry
Acids are commonly used in food processing and preservation.
- Tartaric acid is added to baking powder to help baked goods rise.
- Acetic acid (vinegar) is used in pickling to preserve food.
- Citric acid enhances flavour in soft drinks and sweets.
Some natural acids, such as lactic acid and gastric acid, aid digestion by breaking down food in the stomach.
3. Batteries and Energy Storage
Acids play a crucial role in battery production.
- Sulphuric acid is used in car batteries to enable the chemical reaction that produces electricity.
- Acid salts like ammonium chloride are used in dry cell batteries.
These batteries help power everyday items, from torches to vehicles.
Uses of Bases
Bases are substances that neutralise acids, often producing ionic compounds called salts. Some common examples of bases include:
1. Soap and Cleaning Products
Many bases are found in household cleaning products, helping to remove grease and dirt.
- Sodium hydroxide is used in soap-making and oven cleaners.
- Baking soda (sodium bicarbonate) acts as a mild base for removing odours.
These bases react with fat and oil, making them soapy and easier to wash away.
2. Medicine (Antacids)
Bases play a crucial role in medicine, particularly in treating acidity.
- Antacids like magnesium hydroxide neutralise excess gastric acid in the stomach.
- Sodium bicarbonate is used to relieve acid reflux.
These substances help neutralise acids and reduce discomfort caused by indigestion.
3. Agriculture (Lime in Soil)
Farmers use bases to reduce soil acidity, improving crop growth.
- Calcium hydroxide (lime) neutralises acidic soils.
- Ammonium hydroxide is added to fertilisers to enhance plant health.
This process ensures that plants absorb essential nutrients more efficiently.
How Are Salts Formed? (Neutralisation Reaction Explained)
Salts are an important class of ionic compounds formed when an acid and a base react. This process is known as neutralisation, where the acidic and basic properties cancel each other out, producing a salt and water.
The formation of salts plays a crucial role in chemistry, industry, and daily life, as different salts have various applications.
What Is a Neutralisation Reaction?
A neutralisation reaction occurs when an acid reacts with a base, leading to the formation of a salt and water. This reaction is important in controlling acidity and alkalinity in various applications, from medicine to agriculture.
General Equation for Neutralisation
Acid + Base → Salt + Water
For example:
Hydrochloric acid (HCl) + Sodium hydroxide (NaOH) → Sodium chloride (NaCl) + Water (H₂O)
This reaction shows how a strong acid (hydrochloric acid) neutralises a strong base (sodium hydroxide) to produce sodium chloride (table salt) and water.
How Do Acids and Bases React to Form Salts?
The neutralisation of acids occurs when hydrogen ions (H⁺) from the acid combine with hydroxide ions (OH⁻) from the base to form water (H₂O). The remaining ions from the acid and base create the salt.
Example Reactions
- Sulphuric acid + Sodium hydroxide → Sodium sulphate + Water H2SO4 + 2NaOH → Na2SO4 + 2H2O
- Nitric acid + Potassium hydroxide → Potassium nitrate + Water HNO3 + KOH → KNO3 + H2O
- Acetic acid + Ammonium hydroxide → Ammonium acetate + Water CH3COOH + NH4OH → CH3COONH4 + H2O
Each reaction results in the formation of a neutral salt and water, balancing the acidic and basic properties.
Common Salts and Their Uses
Salts are widely used in cooking, medicine, agriculture, and industry. Here are some common salts and their applications:
| Salt | Chemical Formula | Uses |
|---|---|---|
| Sodium chloride (table salt) | NaCl | Used in cooking, food preservation, and medical treatments. |
| Sodium bicarbonate (baking soda) | NaHCO₃ | Used in baking, cleaning, and as an antacid. |
| Ammonium chloride | NH₄Cl | Used in dry cell batteries and fertilisers. |
| Calcium carbonate | CaCO₃ | Found in limestone, used in cement and antacids. |
| Potassium nitrate | KNO₃ | Used in fertilisers and fireworks. |
| Magnesium sulphate (Epsom salt) | MgSO₄ | Used in medicine and agriculture. |
How Are Salts Used in Everyday Life?
Salts have various practical applications beyond food seasoning. Some examples include:
- Cooking: Sodium chloride enhances flavour and preserves food.
- Medicine: Sodium bicarbonate is used as an antacid to neutralise stomach acidity.
- Agriculture: Ammonium chloride and potassium nitrate improve soil fertility.
- Industrial Uses: Calcium carbonate is used in cement, glass production, and construction.
- Household Cleaning: Baking soda removes stains and neutralises odours.
What Are the Different Types of Salts?
Salts are ionic compounds formed when an acid and a base react in a neutralisation reaction. They are commonly found in household products, food, and industrial processes. Depending on their formation and chemical composition, salts can be classified into normal salts, acidic salts, and basic salts.
Normal Salts
Normal salts are formed when an acid completely neutralises a base, leaving no extra hydrogen (H⁺) or hydroxide (OH⁻) ions. These salts are typically neutral in nature and do not affect the pH scale.
Examples of Normal Salts:
- Sodium chloride (NaCl): Common table salt, used in cooking and food preservation.
- Potassium nitrate (KNO₃): Used in fertilisers and fireworks.
- Calcium sulphate (CaSO₄): Found in plaster of Paris and cement.
How Are Normal Salts Formed?
When hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), it produces sodium chloride (NaCl) and water:
HCl + NaOH → NaCl + H2O
This is a neutralisation reaction, as the acid and base completely react, forming a salt and water.
Acidic Salts
Acidic salts form when a polyprotic acid (an acid with more than one hydrogen ion) is only partially neutralised by a base. This means that some H⁺ ions remain, making the salt acidic in nature.
Examples of Acidic Salts:
- Sodium bisulphate (NaHSO₄): Used in cleaning agents and pH control in swimming pools.
- Ammonium chloride (NH₄Cl): Found in fertilisers and dry cell batteries.
- Potassium bisulphate (KHSO₄): Used in the food industry as an acidity regulator.
How Are Acidic Salts Formed?
When sulphuric acid (H₂SO₄) reacts with sodium hydroxide (NaOH), but not all hydrogen ions are replaced, it forms sodium bisulphate (NaHSO₄):
H2SO4 + NaOH → NaHSO4 + H2O
Since not all hydrogen ions are neutralised, the salt remains acidic in an aqueous solution.
Basic Salts
Basic salts form when a weak acid reacts with a strong base, and some hydroxide ions (OH⁻) remain in the solution, making the salt basic (alkaline) in nature.
Examples of Basic Salts:
- Sodium carbonate (Na₂CO₃): Used in laundry detergents and glass production.
- Calcium bicarbonate (Ca(HCO₃)₂): Found in hard water and responsible for lime scale formation.
- Magnesium hydroxide (Mg(OH)₂): Used as an antacid to neutralise stomach acidity.
How Are Basic Salts Formed?
When sodium hydroxide (NaOH) reacts with carbonic acid (H₂CO₃), it forms sodium carbonate (Na₂CO₃), which is a basic salt:
H2CO3 + 2NaOH → Na2CO3 + 2H2O
Since the base is stronger than the acid, the remaining hydroxide ions make the solution alkaline.
Read more GCSE Chemistry Revision Tips for UK Students
FAQ’s
What is the strongest acid?
Fluoroantimonic acid (HSbF6) is the strongest acid, millions of times stronger than sulfuric acid, dissolving almost anything.
What happens if you mix acids and bases?
They neutralize, forming water and salt, sometimes releasing heat or gas, depending on the strength of reactants.
Why is pH important in daily life?
pH affects health, food, water quality, agriculture, and industry, ensuring safety, effectiveness, and proper function in daily life.
How is table salt different from other salts?
Table salt (sodium chloride) is common, while other salts have different compositions, properties, and uses in chemistry and industry.