Want to understand what mass means in chemistry? This guide will make it simple.
Mass tells us how much matter is in a substance. It helps in making compounds, measuring substances, and getting correct lab results.
In this guide, you will learn what mass really means in chemistry. We’ll also look at the different types of mass and how each one helps in science.
Let’s begin by understanding the basic idea of mass and why it matters so much in chemistry.
Mass in Chemistry
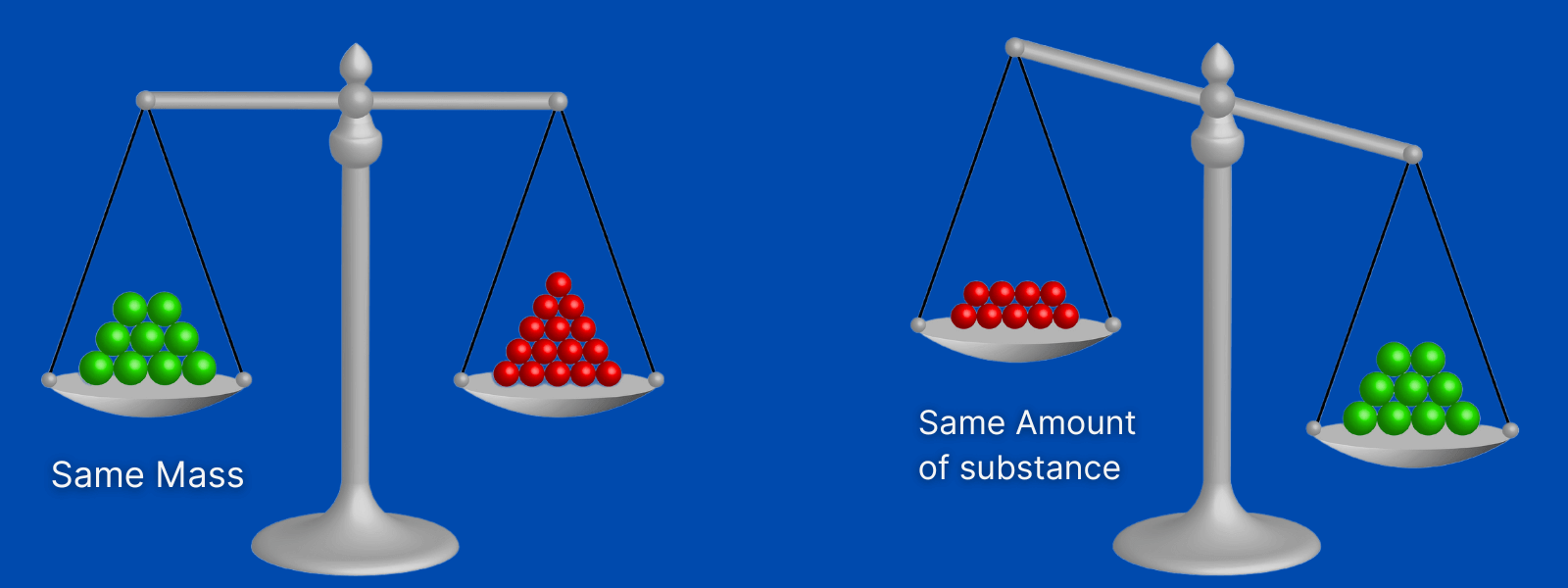
"In chemistry, mass is the measure of the amount of matter in a substance. It determines how much "stuff" makes up an object or sample."
Mass helps chemists quantify substances, making it easier to perform accurate experiments.
SI unit of mass,
The SI unit of mass is the kilogram (kg), although grams (g) are often used in chemistry due to their smaller scale.
Mass remains constant, unaffected by location or gravity, unlike weight. Explore our guide to understand the difference between mass and weight.
To calculate mass, chemists often use the formula:
Mass (m) = Density (ρ) × Volume (V)
Types of mass in chemistry
These are the types of mass in chemistry commonly studied.
- Atomic Mass
- Molar Mass
- Molecular Mass
- Formula Mass
- Isotopic Mass
Atomic mass
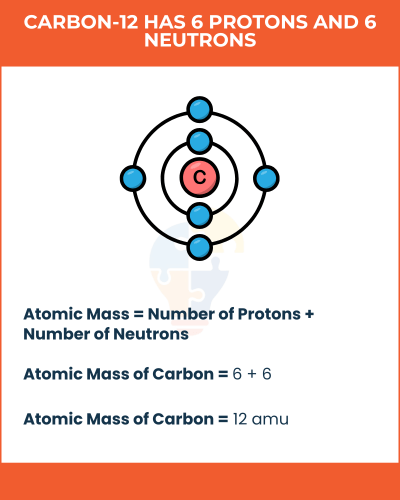
"Atomic mass is the mass of an individual atom, primarily made up of its protons and neutrons."
The formula for atomic mass is straightforward:
Atomic Mass = Number of Protons + Number of Neutrons
For example,
carbon-12 has 6 protons and 6 neutrons. Using the formula, we find:
Atomic Mass of Carbon = 6 + 6 = 12 amu
Molar Mass
Molar mass is the mass of one mole of a substance, measured in grams per mole (g/mol). It represents the mass of Avogadro's number (about 6.022×1023) of particles, such as atoms or molecules.
The formula for molar mass is:
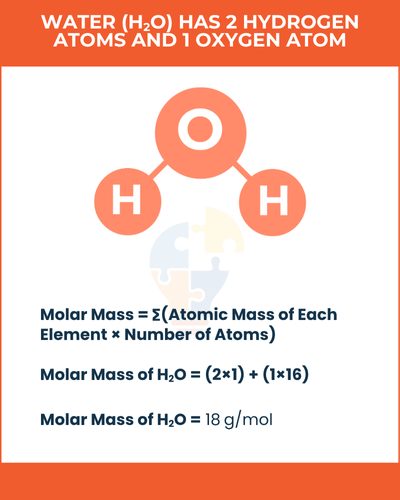
Molar Mass = ∑(Atomic Mass of Each Element × Number of Atoms)
For example,
water (H2O) has 2 hydrogen atoms and 1 oxygen atom. The atomic mass of hydrogen is 1 g/mol, and oxygen is 16 g/mol.
So, Molar Mass of H2O = (2×1) + (1×16)= 18 g/mol
Molecular Mass
"Molecular mass is the mass of a single molecule of a compound, measured in atomic mass units (amu)."
It is the sum of the atomic masses of all the atoms in a molecule. Molecular mass provides insight into the weight of a compound at the molecular level.
The formula for molecular mass is similar to that of molar mass:
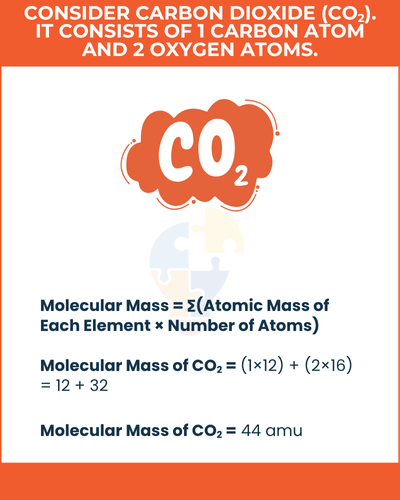
Molecular Mass = ∑(Atomic Mass of Each Element × Number of Atoms)
For example,
consider carbon dioxide (CO2). It consists of 1 carbon atom and 2 oxygen atoms. The atomic mass of carbon is 12 amu, and oxygen is 16 amu. Therefore, the calculation is:
Molecular Mass of CO2 = (1×12) + (2×16) = 12 + 32 = 44 amu
Molecular mass is crucial for understanding the properties of compounds and predicting their behavior in chemical reactions.
Formula Mass
"Formula mass is the mass of one formula unit of an ionic compound, measured in atomic mass units (amu)."
It is calculated by adding the atomic masses of all the atoms in the empirical formula of the compound. Formula mass is primarily used for ionic compounds, as they don’t form discrete molecules like covalent compounds.
Formula:
∑ (Atomic Mass of Each Element × Number of Atoms in the formula)
For Example:
Consider sodium chloride (NaCl). It consists of 1 sodium atom and 1 chlorine atom.
The atomic mass of sodium (Na) is 23 amu, and chlorine (Cl) is 35.5 amu.
So, Formula Mass of NaCl = (1×23) + (1×35.5) = 23 + 35.5 = 58.5 amu
Formula mass helps understand the properties of ionic compounds and their behavior in reactions.

Isotopic Mass
“Isotopic mass is the mass of a specific isotope of an element, measured in atomic mass units (amu).”
Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons, resulting in different masses. Isotopic mass helps in identifying and studying the properties of various isotopes.
The formula for isotopic mass is straightforward,
As it is simply the mass of the individual isotope itself. There isn’t a specific formula to calculate it since isotopic mass is usually provided in tables or measured experimentally.
For example,
consider the isotope carbon-14 (C14). It has 6 protons and 8 neutrons. The isotopic mass of carbon-14 is approximately 14.003 amu. This specific mass differs from that of carbon-12, which is about 12.000 amu.
Isotopic mass is important in fields like radiocarbon dating and nuclear medicine, where specific isotopes play critical roles.
What are Avogadro’s Numbers ?
Avogadro's number is a fundamental constant in chemistry, representing the number of particles in one mole of a substance. It is approximately 6.022×1023 particles, which can be atoms, molecules, ions, or other entities.
This number allows chemists to convert between the mass of a substance and the number of particles it contains.
For example, if you have one mole of carbon atoms, it contains about 6.022×1023 carbon atoms.
Avogadro’s number is essential for stoichiometry, helping to calculate reactants and products in chemical reactions.
It also plays a vital role in understanding the relationship between mass and the number of particles, making it a cornerstone of molecular chemistry.
Practical Tip: How to Avoid Mistakes
Many students lose marks by:
- Confusing Mr and Ar
- Forgetting units (g/mol)
- Using average mass incorrectly
- Skipping steps in calculations
Always write:
- Formula
- Substitution
- Units
This structure wins marks.
Why Choose Tutorhelpme Chemistry Tutors
- Expert Knowledge: Chemistry tutors possess in-depth knowledge of complex concepts, ensuring students grasp difficult topics easily.
- Personalized Learning: Tutors tailor lessons to fit individual learning styles and needs, promoting better understanding.
- Improved Grades: With focused guidance, students often see significant improvements in their grades and exam scores.
- Exam Preparation: Tutors provide targeted support for upcoming tests, helping students develop effective study strategies.
- Enhanced Confidence: Working with a tutor boosts confidence, making students more willing to tackle challenging problems.
- Flexible Scheduling: Tutors can accommodate busy schedules, providing sessions that fit students’ availability.
Read More What is a mass in physics?
What is the difference between atomic mass and molecular mass?
Atomic mass is the mass of a single atom, while molecular mass is the total mass of all atoms in a molecule.
Learn More Difference Between Molar mass and Molecular mass
Why is molar mass important in chemistry?
Molar mass helps chemists calculate the amount of a substance in moles, essential for stoichiometry and chemical reactions.
What does isotopic mass represent?
Isotopic mass is the mass of a specific isotope of an element, reflecting differences in neutron numbers among isotopes.