States of matter describe the physical forms in which substances exist. The main states are solid, liquid, gas, and plasma, each with unique properties based on particle arrangement and energy levels.
Understanding states of matter is essential in science and daily life. From water changing forms to gases in the air, these concepts explain natural and industrial processes.
Scientists use this knowledge to develop new materials, while industries apply it in food production, medicine, and engineering. Recognizing how matter behaves helps in everyday tasks like cooking, freezing, and even breathing.
What Are the Different States of Matter?
Matter exists in different states, each with unique properties based on particle arrangement and energy levels. The five main states of matter are:
Solid
In a solid, particles are tightly packed and arranged in a fixed pattern. This structure gives solids a definite shape and volume.
They do not flow like liquids or expand like gases. Examples include ice, wood, and metals.

Liquid
Liquids have loosely packed particles that move freely, allowing them to take the shape of their container while maintaining a fixed volume.
Water, oil, and milk are common examples. Unlike solids, liquids can flow and be poured.

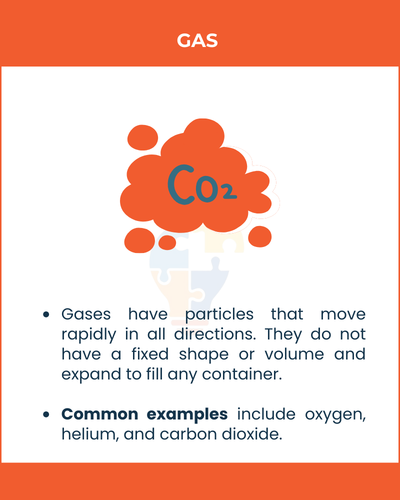
Gas
Gases have particles that move rapidly in all directions. They do not have a fixed shape or volume and expand to fill any container.
Common examples include oxygen, helium, and carbon dioxide.
Plasma
Plasma is a highly energized state where electrons separate from atoms, creating charged particles. This state is found in stars, lightning, and neon signs.
Plasma is different from gases because it conducts electricity and reacts to magnetic fields.
Bose-Einstein Condensate
Bose-Einstein Condensate (BEC) forms at extremely low temperatures, close to absolute zero. In this state, atoms lose their individuality and behave as a single quantum entity. Scientists use BEC to study quantum mechanics and superfluidity.
What Are the Properties of Solids, Liquids, and Gases?
Each state of matter has unique properties that define how it behaves. Understanding these properties helps explain their roles in daily life and science.
Properties of Solids
Solids have a fixed shape and volume due to strong molecular bonds.
- Definite Shape: Solids do not change shape unless forced.
- Fixed Volume: The amount of space a solid takes up does not change.
- High Density: Particles are tightly packed, making solids heavier.
- Strong Intermolecular Forces: Particles stay in a fixed position.
- Incompressibility: Solids cannot be compressed easily.
Properties of Liquids
Liquids take the shape of their container but maintain a constant volume.
- No Fixed Shape: Liquids flow and adapt to the container’s shape.
- Fixed Volume: The amount of liquid remains the same unless spilled.
- Moderate Density: Particles are less tightly packed than in solids.
- Weaker Intermolecular Forces: Particles can move but stay close together.
- Slight Compressibility: Liquids can be slightly compressed under pressure.
Properties of Gases
Gases have no fixed shape or volume and expand to fill their container.
- No Fixed Shape: Gases spread out in all directions.
- No Fixed Volume: Gases expand or compress based on the container size.
- Low Density: Particles are far apart, making gases lighter.
- Weak Intermolecular Forces: Particles move freely at high speed.
- High Compressibility: Gases can be compressed into smaller spaces.
How Do Solids, Liquids, and Gases Differ?
Each state of matter differs in shape, volume, particle arrangement, and movement.
Solids vs. Liquids
- Solids have a fixed shape, while liquids take the shape of their container.
- Solids have strong molecular bonds, while liquids have weaker ones.
- Solids do not flow, but liquids can move and pour easily.
Liquids vs. Gases
- Liquids have a fixed volume, but gases expand to fill any space.
- Liquids have moderate density, while gases have low density.
- Liquids are slightly compressible, but gases are highly compressible.
Solids vs. Gases
- Solids have strong molecular forces, while gases have almost none.
- Solids are dense and rigid, while gases are light and spread out.
- Solids do not change shape or volume, but gases expand freely.
How Do States of Matter Change?
Matter changes state when energy is added or removed. These changes occur due to temperature and pressure variations, altering how particles move and interact. The main phase changes include:
Melting – Solid to Liquid
Melting happens when a solid absorbs heat, causing its particles to move more freely. Once the temperature reaches the melting point, the solid turns into a liquid.
An example is ice melting into water.
Freezing – Liquid to Solid
Freezing occurs when a liquid loses heat, slowing down particle movement until they form a fixed structure. When water reaches 0°C, it freezes into ice. This process is also called solidification.
Evaporation – Liquid to Gas
Evaporation takes place when a liquid gains energy, allowing its particles to escape as gas. It happens at the surface of a liquid, even below the boiling point.
Examples include water evaporating from wet clothes or ponds.
Condensation – Gas to Liquid
Condensation occurs when a gas loses heat, causing its particles to slow down and form a liquid. This process is seen when water droplets form on a cold surface or when steam condenses on a mirror.
Sublimation – Solid to Gas
Sublimation happens when a solid turns directly into a gas without becoming a liquid first. This occurs when a solid absorbs enough energy to break its particle bonds. Dry ice (solid carbon dioxide) sublimates into gas without melting.
Deposition – Gas to Solid
Deposition is the reverse of sublimation. It happens when a gas transforms into a solid without becoming a liquid. An example is frost forming on cold surfaces when water vapor in the air freezes directly into ice.
How Do Temperature and Pressure Affect States of Matter?
Temperature and pressure play a crucial role in changing the state of matter. These factors determine how particles move, interact, and transition between different states.
How Heat Causes State Changes
Heat increases the energy of particles, making them move faster and break free from their fixed positions. This leads to:
- Melting – A solid turns into a liquid as heat weakens particle bonds.
- Evaporation & Boiling – A liquid changes into a gas when particles gain enough energy to escape.
- Sublimation – Some solids, like dry ice, turn directly into gas when heated.
Removing heat has the opposite effect:
- Freezing – A liquid loses energy, causing particles to slow down and form a solid.
- Condensation – A gas cools, reducing particle movement and forming a liquid.
- Deposition – A gas turns into a solid when heat is removed rapidly.
“Get a sucess in your exams with our 1-on-1 online GCSE chemistry tutors.”
Effect of High and Low Pressure
Pressure affects how closely particles are packed together. Changes in pressure can alter the state of matter even without temperature changes.
- High Pressure:
- Increases particle density, forcing gases into liquids and liquids into solids.
- Example: Carbon dioxide turns into dry ice under high pressure.
- Low Pressure:
- Reduces particle interaction, making solids and liquids turn into gases more easily.
- Example: Water boils at a lower temperature at high altitudes due to reduced pressure.
What Are the Applications of Different States of Matter?
The different states of matter play a vital role in daily life, industries, and scientific research. Understanding their applications helps in technology, manufacturing, and scientific advancements.
Everyday Examples of Solids, Liquids, and Gases
- Solids: Used in construction (bricks, metals), packaging (plastic, cardboard), and household objects (furniture, electronics).
- Liquids: Essential for drinking water, cooking oil, fuels (petrol, diesel), and cleaning products.
- Gases: Used in breathing (oxygen), cooking (natural gas), and transportation (compressed air in tires).
Industrial Uses of Plasma
Plasma is widely used in industries due to its high energy and unique properties. Some applications include:
- Electronics: Plasma is used in semiconductor manufacturing and plasma screens.
- Medical Treatments: Plasma sterilization is used in hospitals to kill bacteria.
- Space and Energy: Plasma-based fusion research aims to develop clean energy sources.
Supercooled Materials and Bose-Einstein Condensates
- Supercooled Materials: Used in superconductors, which allow electricity to flow without resistance, improving energy efficiency in technology.
- Bose-Einstein Condensates (BEC): Help scientists study quantum mechanics and are used in precision measurement tools like atomic clocks.
What Are Some Unusual States of Matter?
Beyond the common states, matter can exist in unusual forms under extreme conditions. These states have unique properties that challenge our understanding of physics.
What Is a Superfluid?
A superfluid is a liquid that flows without friction. When cooled to extremely low temperatures, some substances, like liquid helium, become superfluids.
This means they can move through tiny spaces without resistance, climb walls, and never settle at rest. Superfluids are studied for their potential in advanced cooling systems and quantum mechanics.
How Do Liquid Crystals Work?
Liquid crystals are a unique state of matter that has properties of both liquids and solids. Their molecules flow like a liquid but maintain some ordered structure like a solid.
These properties make them ideal for LCD screens, where they control light to create clear images in TVs, smartphones, and monitors.
Exotic States in Extreme Conditions
- Quark-Gluon Plasma: Found in the early universe and recreated in particle colliders, this state consists of free quarks and gluons.
- Time Crystals: A new state of matter where particles move in a repeating pattern over time without external energy.
- Rydberg Matter: A state where atoms are highly excited and interact in unusual ways, studied for space science and quantum computing.
Real-Life Examples of States of Matter
States of matter can be observed in everyday activities, from cooking to nature. These examples help us understand how solids, liquids, and gases interact in daily life.
How Do We See States of Matter in Cooking?
Cooking involves various state changes:
- Solid to Liquid: Butter melts in a hot pan.
- Liquid to Gas: Water boils and turns into steam.
- Liquid to Solid: Cake batter solidifies when baked.
Water Cycle (Ice, Water, Steam)
The water cycle shows natural state changes:
- Ice (Solid): Forms in cold temperatures, like snow and glaciers.
- Water (Liquid): Fills rivers, lakes, and oceans.
- Steam (Gas): Rises from boiling water and condenses to form clouds.
Air in Balloons and Tires
Gases take the shape of their container:
- Balloons: Air expands inside, keeping the balloon inflated.
- Tires: Compressed air gives structure and support to vehicles.
Read more What is Chemistry? Branches of Chemistry and Daily Life Exmaples
FAQ’s
How is plasma different from gas?
Plasma has charged particles and conducts electricity, while gas has neutral particles and does not conduct electricity.
Where can plasma be found in nature?
Plasma exists in stars, lightning, auroras, and the sun’s corona, making it the most abundant state in the universe.
How does viscosity affect liquids?
Viscosity determines a liquid’s flow; high viscosity means slow movement (honey), while low viscosity allows fast flow (water).
Can a liquid become a solid and gas at the same time?
No, a liquid cannot become a solid and gas at the same time under normal conditions; however, at a specific temperature and pressure called the “triple point,” a substance can exist in all three phases (solid, liquid, and gas) simultaneously.