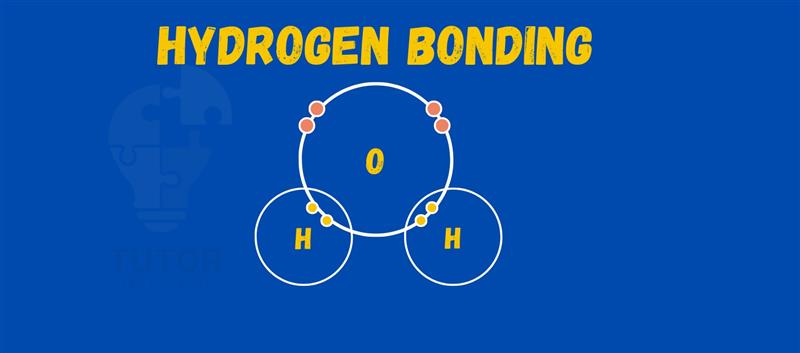
Hydrogen bonds are vital in many chemical and biological processes.
These bonds form when a hydrogen atom, bonded to a highly electronegative atom like oxygen or nitrogen, interacts with another electronegative atom.
This interaction creates unique properties such as high boiling points and strong cohesion.
In this blog, explore the properties and types of hydrogen bonds, understanding their crucial role in water, proteins, and DNA.
You’ll see how these bonds influence everything from the structure of ice to the stability of our genetic material.
What Are Hydrogen Bonds?
A hydrogen bond is a weak attraction between a hydrogen atom bonded to a highly electronegative atom and another electronegative atom nearby.
It is not a true chemical bond like ionic or covalent bonds. It is an intermolecular force that helps molecules stick together.
Formation of Hydrogen Bonds
Hydrogen bonds form through the following steps:
- Presence of Highly Electronegative Atom: Hydrogen atom must be bonded to a strong atom like oxygen, nitrogen, or fluorine.
- Partial Charges: The electronegativity of the atom to which hydrogen is bonded creates a partial positive charge on the hydrogen atom and a partial negative charge on the electronegative atom.
- Attraction: The partial positive charge on the hydrogen atom is attracted to the partial negative charge on another electronegative atom, leading to the formation of a hydrogen bond.
Properties of Hydrogen Bonds
Hydrogen bonds exhibit several unique properties that significantly influence the behavior of molecules and compounds.
- Strength: While hydrogen bonds are weaker than covalent bonds, they are stronger than van der Waals forces. The strength of hydrogen bonds varies but typically ranges from 5 to 30 kJ/mol.
- Directionality: Hydrogen bonds are highly directional. They form in a straight line between the hydrogen atom and the electronegative atom it interacts with. This directionality contributes to the specific geometric arrangements in molecules.
- Bond Length: The length of a hydrogen bond is longer than a covalent bond but shorter than van der Waals interactions. The bond length typically ranges from 1.5 to 2.5 Å (angstroms).
- Influence on Physical Properties: Hydrogen bonds significantly influence the physical properties of substances. They contribute to higher boiling and melting points, increased solubility, and unique structural characteristics.
- Impact on Molecular Structures: Hydrogen bonds play a crucial role in determining the shapes and structures of complex molecules, such as proteins and nucleic acids.
Read more what are bonds? types of bonds
Types of Hydrogen Bonds
Hydrogen bonds can be classified into two main types based on their occurrence:
Intermolecular Hydrogen Bonds
These bonds form between different molecules.
Example,
The hydrogen bonds between water molecules are a classic example. Each water molecule (H₂O) can form hydrogen bonds with four neighboring water molecules.
The oxygen atom in one water molecule, which has a partial negative charge, attracts the hydrogen atom in another water molecule, which has a partial positive charge.
This bonding results in water’s unique properties, such as high boiling and melting points, surface tension, and specific heat capacity.
Intramolecular Hydrogen Bonds
These bonds form within a single molecule.
Example,
In proteins, intramolecular hydrogen bonds play a crucial role in maintaining their secondary and tertiary structures.
For instance, in an alpha-helix structure, the hydrogen bonds form between the carbonyl oxygen of one amino acid and the amide hydrogen of another, four residues apart.
This bonding stabilizes the helical structure, making it resistant to denaturation.
Similarly, in beta-sheets, hydrogen bonds form between carbonyl oxygens and amide hydrogens of adjacent polypeptide chains, providing structural stability.
Importance of Hydrogen Bonds in Water
Water’s remarkable properties can largely be attributed to hydrogen bonds. Here are some key points:
- High Boiling and Melting Points: Water has unusually high boiling and melting points for a molecule of its size due to hydrogen bonding. This property is essential for maintaining liquid water in a wide range of temperatures on Earth.
- Surface Tension: Hydrogen bonds cause high surface tension in water, making it capable of forming droplets and allowing small insects to walk on its surface.
- Heat Capacity: Water’s high specific heat capacity is due to hydrogen bonds. It can absorb and release significant amounts of heat without experiencing drastic temperature changes, making it an excellent heat buffer.
- Solvent Properties: Hydrogen bonds make water an excellent solvent for polar and ionic substances. This property is crucial for biological processes, as it allows for the dissolution and transport of various molecules within living organisms.
- Density Anomaly: Hydrogen bonds are responsible for water’s density anomaly, where ice is less dense than liquid water. This phenomenon ensures that ice floats, providing insulation for aquatic life during cold periods.
Importance of Hydrogen Bonds in Proteins
Proteins rely heavily on hydrogen bonds to maintain their structure and function. Here are some ways hydrogen bonds influence proteins:
- Secondary Structure: Hydrogen bonds between the backbone atoms of amino acids stabilize secondary structures like alpha-helices and beta-sheets. In alpha-helices, hydrogen bonds form between the carbonyl oxygen of one amino acid and the amide hydrogen of another, four residues apart. In beta-sheets, hydrogen bonds form between carbonyl oxygens and amide hydrogens of adjacent polypeptide chains.
- Tertiary Structure: Hydrogen bonds between side chains of amino acids contribute to the overall folding and three-dimensional structure of proteins. These interactions help maintain the protein’s stability and functionality.
- Enzyme Activity: Hydrogen bonds play a crucial role in the active sites of enzymes, helping to stabilize the transition states of chemical reactions and facilitating substrate binding.
- Protein-Protein Interactions: Hydrogen bonds are involved in protein-protein interactions, which are essential for various biological processes, such as signal transduction and cellular communication.
Impact of Hydrogen Bonds on DNA
Hydrogen bonds are vital for the stability and function of DNA, the molecule that carries genetic information. Here’s how they contribute:
- Double-Helix Structure: Hydrogen bonds between complementary base pairs (adenine-thymine and guanine-cytosine) hold the two strands of the DNA double helix together. Adenine forms two hydrogen bonds with thymine, while guanine forms three hydrogen bonds with cytosine.
- Replication and Transcription: Hydrogen bonds allow the DNA strands to separate and reanneal during replication and transcription. This reversible nature of hydrogen bonds ensures the accuracy and efficiency of these processes.
- Stability: The hydrogen bonds between base pairs contribute to the overall stability of the DNA molecule, protecting the genetic information from damage and mutation.
- Gene Regulation: Hydrogen bonds are involved in the recognition and binding of regulatory proteins to specific DNA sequences, playing a crucial role in gene expression and regulation.
Applications of Hydrogen Bonds in Industry
Hydrogen bonds have several practical applications in various industries:
- Textile Industry: Hydrogen bonds play a crucial role in the dyeing and finishing of textiles. The interaction between dye molecules and fibers, such as cotton and wool, involves hydrogen bonding, ensuring proper attachment and colorfastness.
- Pharmaceuticals: Hydrogen bonds are essential in drug design and development. They influence the binding affinity and specificity of drugs to their target molecules, such as enzymes and receptors. Understanding hydrogen bonding helps in designing more effective and selective drugs.
- Food Industry: Hydrogen bonds affect the texture and stability of food products. For instance, the gelation of gelatin and the formation of gluten networks in dough involve hydrogen bonding, influencing the final product’s texture and quality.
- Materials Science: Hydrogen bonds are utilized in the development of advanced materials, such as hydrogels and shape-memory polymers. These materials have applications in drug delivery, tissue engineering, and responsive coatings.
Hydrogen Bonds in Everyday Life
Hydrogen bonds impact our daily lives in various ways:
- Ice and Snow: The formation of ice and snow is a result of hydrogen bonding between water molecules. This phenomenon affects weather patterns, ecosystems, and human activities.
- Personal Care Products: Hydrogen bonds are involved in the formulation of personal care products, such as shampoos, conditioners, and lotions. They influence the texture, consistency, and effectiveness of these products.
- Cleaning Agents: Hydrogen bonds play a role in the effectiveness of cleaning agents. For instance, hydrogen bonding between water molecules and dirt particles helps in the removal of dirt and grime during cleaning processes.
- Taste and Smell: Hydrogen bonds are involved in the perception of taste and smell. The interaction between odorant or flavor molecules and receptors in the nose or mouth often involves hydrogen bonding, influencing our sensory experiences.
Advances in Hydrogen Bond Research
Research on hydrogen bonds continues to evolve, leading to new discoveries and applications.
Some recent advances include:
- Supramolecular Chemistry: Hydrogen bonds are fundamental in the field of supramolecular chemistry, where they are used to create complex and functional molecular assemblies. These assemblies have applications in drug delivery, catalysis, and nanotechnology.
- Hydrogen Bonding in Materials Science: Researchers are exploring the role of hydrogen bonds in developing new materials with unique properties, such as self-healing materials, responsive surfaces, and advanced adhesives.
- Biomolecular Simulations: Advances in computational methods and biomolecular simulations have provided deeper insights into the role of hydrogen bonds in biological processes. These simulations help in understanding protein folding, enzyme mechanisms, and drug interactions.
- Hydrogen Bonds in Renewable Energy: Hydrogen bonds are being studied in the context of renewable energy sources, such as hydrogen fuel cells and biofuels. Understanding hydrogen bonding helps in improving the efficiency and performance of these energy technologies.
Common GCSE Mistakes About Hydrogen Bonds
Students often:
- Call hydrogen bonds “true bonds” (they are forces)
- Mix them up with ionic/covalent bonds
- Forget that they occur between molecules, not inside them
- Apply them to molecules without H–O, H–N, or H–F
Fixing these mistakes improves extra marks.
Read More What Are Metallic Bonds? Examples, Properties and Formation
FAQs
What is a hydrogen bond?
A hydrogen bond is a weak attraction between a hydrogen atom bonded to an electronegative atom and another electronegative atom.
Which atoms are needed for hydrogen bonding?
Hydrogen must bond to oxygen, nitrogen, or fluorine.
Are hydrogen bonds strong?
They are weaker than ionic or covalent bonds but stronger than most van der Waals forces.
Why does water have a high boiling point?
Because hydrogen bonds hold water molecules together, more energy is needed to separate them.
Is a hydrogen bond a chemical bond?
No. It is an intermolecular force, not a chemical bond.