Electron configuration shows how electrons arrange themselves in an atom. It helps explain chemical properties, reactivity, and periodic table trends.
Elements with full outer shells are stable, while others react to achieve stability. This concept helps predict how elements bond and form compounds.
Understanding electron configuration is key to learning how atoms behave in chemistry and real-world applications.
What Is Electron Configuration?
Electron configuration describes how electrons are arranged in an atom's orbitals. It follows specific rules that determine how electrons fill energy levels and subshells around the nucleus.
Understanding electron configuration helps explain chemical properties, bonding, and reactivity.
Every atom has a unique electronic configuration, which depends on its atomic number. This configuration determines how elements interact in chemical reactions and their position in the periodic table.
How Are Electrons Arranged in Atoms?
Electrons are arranged in energy levels (shells) around the nucleus. These electron shells contain subshells called s, p, d, and f orbitals, which hold electrons in a specific order.
Energy Levels and Shells
Each energy level (or electron shell) can hold a maximum number of electrons based on the formula 2n², where n is the principal quantum number (shell number).
| Shell (Energy Level) | Maximum Number of Electrons |
|---|---|
| First Shell (n = 1) | 2 |
| Second Shell (n = 2) | 8 |
| Third Shell (n = 3) | 18 |
| Fourth Shell (n = 4) | 32 |
For example, a neutral atom of chlorine (Cl) has 17 electrons, which are arranged as 1s² 2s² 2p⁶ 3s² 3p⁵.
Subshells and Orbitals
Each shell contains different subshells:
| Subshell | Maximum Electrons | Orbital Shape |
|---|---|---|
| s | 2 | Spherical |
| p | 6 | Dumbbell-shaped |
| d | 10 | Complex shapes |
| f | 14 | Even more complex |
For example, in the third shell, electrons fill the 3s, 3p, and 3d orbitals in a specific order.
Why Is Electron Configuration Important?
- It explains the chemical properties and reactivity of elements.
- It helps predict bond formation and ionisation energy.
- It determines the placement of elements in the periodic table.
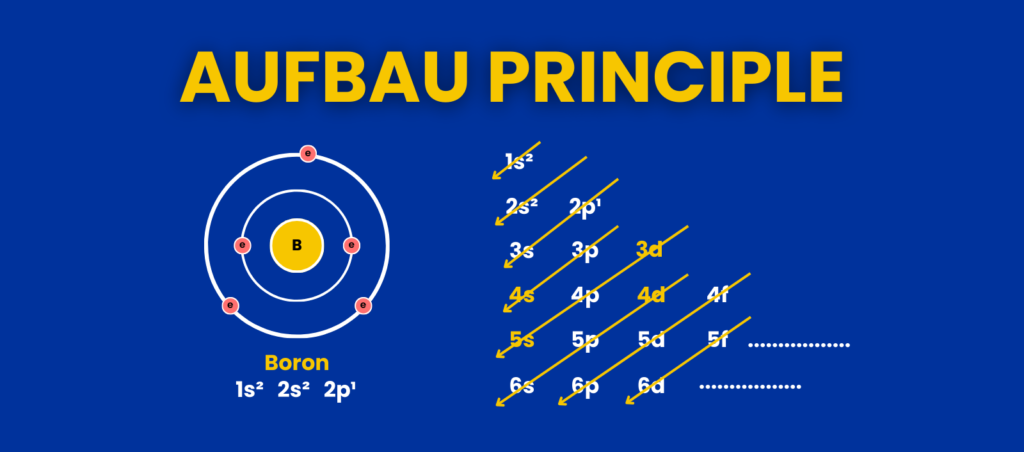
What Is the Aufbau Principle?
Electrons follow a specific order of increasing energy when filling orbitals. The sequence starts with 1s (lowest energy) and continues as follows:
1s→ 2s → 2p → 3s→ 3p → 4s → 3d → 4p → 5s → 4d → 5p → 6s → 4f → 5d → 6p →7s
This order is based on the energy levels of the orbitals. Lower-energy orbitals fill before higher-energy orbitals, ensuring a stable configuration.
Why Does 4s Fill Before 3d?
Although 3d comes before 4s in notation, the 4s orbital has lower energy than the 3d orbital. This is why electrons fill 4s before 3d. However, in transition metals, electrons from 4s may be removed first when forming cations.
Example:
- Potassium (K): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹
- Calcium (Ca): 1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
What Is Hund’s Rule and Pauli’s Principle?
Hund’s Rule: How Electrons Occupy Orbitals
Hund’s Rule states that electrons fill orbitals singly before pairing. This reduces electron repulsion, leading to a more stable configuration.
Example:
In the 2p subshell, electrons first occupy each p orbital singly, then they pair up:
↑ ↑ ↑
Once all orbitals contain one electron, additional electrons start pairing up:
↑ ↓ ↑ ↑
Pauli Exclusion Principle: No Identical Electrons
The Pauli Exclusion Principle states that no two electrons in an atom can have identical quantum states. Each electron has a unique set of quantum numbers, including its spin quantum number.
- If two electrons occupy the same orbital, they must have opposite spins:
↑ ↓
This principle ensures each electron has a distinct position, preventing overlap.
How to Write Electron Configurations?
Electron configuration describes how electrons are arranged in an atom’s orbitals. Scientists use a standard notation to represent this arrangement, helping to predict an element’s chemical properties and reactivity.
Each electron is placed in an energy level or electron shell, following the Aufbau principle, which states that electrons fill lower energy orbitals first before moving to higher ones.
Notation Format for Electron Configuration
Electron configurations follow a specific order of orbitals, written as:
nℓx
Where:
- n = Principal quantum number (energy level).
- ℓ = Orbital type (s, p, d, f).
- x = Number of electrons in that orbital.
For example, the electron configuration of a hydrogen atom is written as 1s¹, meaning one electron is in the first shell’s s orbital.
Examples of Electron Configurations
Oxygen (O) – Atomic Number 8
- Oxygen has 8 electrons.
- The configuration of an element follows the filling order:
Oxygen: 1s22s22p4
Sodium (Na) – Atomic Number 11
- Sodium has 11 electrons.
- The order of increasing orbitals determines placement:
Sodium: 1s22s22p63s1
Iron (Fe) – Atomic Number 26
- Iron has 26 electrons, so electrons occupy the orbitals in this order:
Iron: 1s22s22p63s23p64s23d6

What Is Noble Gas Notation?
Instead of listing all orbitals, chemists use noble gas notation to shorten electron configurations. This method replaces the filled electron shells with the symbol of the previous noble gas.
For example, rather than writing the full electron configuration of calcium, it can be simplified using argon (Ar) as follows:
Calcium (Ca): [Ar]4s2
This notation saves space and makes writing electron configurations easier.
“Get success with our 1-on-1 online GCSE chemistry tutors.”
How Does Electron Configuration Affect Reactivity?
Electron configuration plays a key role in chemical properties and bonding. It determines how an atom reacts with other elements, influencing whether it gains, loses, or shares electrons.
How Electron Configuration Determines Reactivity
The number of electrons in the outermost shell (valence shell) decides how reactive an element is. Atoms with full electron shells are stable, while those with incomplete shells react to achieve a stable configuration.
- Metals (e.g., Sodium, Potassium) lose electrons easily because they have one electron in their outer shell.
- Non-metals (e.g., Chlorine, Oxygen) gain electrons to completely fill their outer shell.
- Noble gases have completely filled electron shells, making them unreactive.
Why Are Group 1 Metals Highly Reactive?
Group 1 metals have one electron in their valence shell. Losing this electron gives them a stable noble gas configuration.
For example, sodium (Na) has the electron configuration 1s² 2s² 2p⁶ 3s¹. It loses one electron to form Na⁺, achieving the stable configuration of neon (Ne):
Na → Na+ + e−
This is why Group 1 metals react vigorously with water and oxygen.
Why Are Noble Gases Stable?
Noble gases have completely filled electron shells, so they do not need to gain or lose electrons. This makes them unreactive.
For example, neon (Ne) has the electron configuration 1s² 2s² 2p⁶. It already has a full outer shell, so it does not form compounds easily.
What Are the Electron Configurations of Ions?
Ions form when atoms gain or lose electrons to achieve a stable electronic structure.
How Do Cations Form?
Cations are positively charged ions that form when atoms lose electrons.
For example, sodium (Na) has one electron in its outer shell. When it loses this electron, it becomes Na⁺:
Na(1s22s22p63s1) → Na+(1s22s22p6) + e−
The Na⁺ ion now has the same electron configuration as neon (Ne), making it stable.
How Do Anions Form?
Anions are negatively charged ions that form when atoms gain electrons.
For example, chlorine (Cl) has seven electrons in its outer shell. It needs one more electron to complete the octet rule. When it gains one electron, it becomes Cl⁻:
Cl(1s22s22p63s23p5)+ e− → Cl−(1s22s22p63s23p6)
The Cl⁻ ion now has the same electron configuration as argon (Ar), making it stable.
How Is Electron Configuration Used in the Periodic Table?
The periodic table is a structured arrangement of elements based on their atomic number and electron configuration. The way electrons occupy orbitals determines an element’s chemical properties and its position in the periodic table. Understanding electron configuration helps explain trends such as atomic size, ionisation energy, and electronegativity.
How Are Elements Arranged in the Periodic Table?
Structure of the Periodic Table
Elements in the periodic table are arranged in periods (rows) and groups (columns) based on their electron configuration. The structure of the periodic table allows scientists to predict the chemical properties of elements.
- Periods (Rows)
- Each period represents a new energy level or electron shell.
- Elements in the same period have the same number of electron shells but an increasing atomic number.
- Example: Sodium (Na) and Chlorine (Cl) belong to Period 3, meaning they both have three electron shells.
- Groups (Columns)
- Elements in the same group have the same number of valence electrons (electrons in the outermost shell).
- These elements share similar chemical properties.
- Example: Group 1 (Alkali Metals) all have one electron in their outermost shell, making them highly reactive.
How Does Electron Configuration Affect Periodic Trends?
Atomic Size (Atomic Radius)
Atomic size is influenced by electron configuration:
- Across a period: Atomic size decreases because electrons are added to the same shell, while the increasing nuclear charge pulls them closer to the nucleus.
- Down a group: Atomic size increases as additional electron shells are added, increasing the distance from the nucleus.
Ionisation Energy
Ionisation energy is the energy required to remove an electron from an atom. Electron configuration influences this trend:
- Across a period: Ionisation energy increases because electrons are closer to the nucleus, making them harder to remove.
- Down a group: Ionisation energy decreases as electrons are further from the nucleus, reducing attraction.
Electronegativity
Electronegativity measures an element’s ability to attract electrons in a bond:
- Across a period: Electronegativity increases because atoms have a greater nuclear charge, pulling electrons more strongly.
- Down a group: Electronegativity decreases as electron shells increase, reducing attraction to bonding electrons.
Read more What Are Alkanes, Alkenes, and Alkynes?
FAQ’s
How do transition metals have variable configurations?
Transition metals have closely spaced 3d and 4s orbitals, allowing electrons to shift, leading to different stable electron arrangements.
How is electron configuration used in spectroscopy?
Spectroscopy analyses electron transitions between energy levels, helping identify elements in stars, forensic science, and chemical research.