Want to learn what makes up fuels and plastics? Start with understanding hydrocarbons.
These are organic compounds made of carbon and hydrogen. They form the base of many things like fuel, plastic, and chemicals.
Now let’s look at the three main types of hydrocarbons. These are alkanes, alkenes, and alkynes, based on how their carbon atoms bond.
Alkanes
Alkanes are saturated hydrocarbons because they contain only single bonds between carbon atoms. They follow the general formula:
CnH2n + 2
- Example: Methane (CH₄), Ethane (C₂H₆)
- Properties:
- Relatively unreactive
- Burn in oxygen to produce carbon dioxide and water
- Used as fuels and lubricants

Now that you know how alkanes work, let’s look at alkenes, which have a key difference in their bonds.
Alkenes
Alkenes are unsaturated hydrocarbons that contain at least one carbon-carbon double bond. They have the general formula:
CnH2n
- Example: Ethene (C₂H₄), Propene (C₃H₆)
- Properties:
- More reactive than alkanes due to the presence of a double bond
- Undergo addition reactions
- Used in plastics and chemical synthesis

Now that you know what makes alkenes different, let’s move on to alkynes, which contain triple bonds between carbon atoms.
Alkynes
Alkynes are unsaturated hydrocarbons that contain at least one carbon-carbon triple bond. Their general formula is:
CnH2n−2
- Example: Ethyne (C₂H₂), Propyne (C₃H₄)
- Properties:
- Highly reactive compared to alkanes and alkenes
- Used in welding (acetylene gas) and organic synthesis

Now that you’ve learned about alkanes, alkenes, and alkynes, let’s see how their structures are different from each other.
How Do Alkanes, Alkenes, and Alkynes Differ in Structure?
The main difference between alkanes, alkenes, and alkynes is the type of bond between carbon atoms. This bond type affects how stable and reactive they are.
| Hydrocarbon Type | Bond Type | Reactivity | Example |
|---|---|---|---|
| Alkanes | Single bonds | Least reactive | Methane (CH₄) |
| Alkenes | One or more double bonds | More reactive | Ethene (C₂H₄) |
| Alkynes | One or more triple bonds | Most reactive | Ethyne (C₂H₂) |
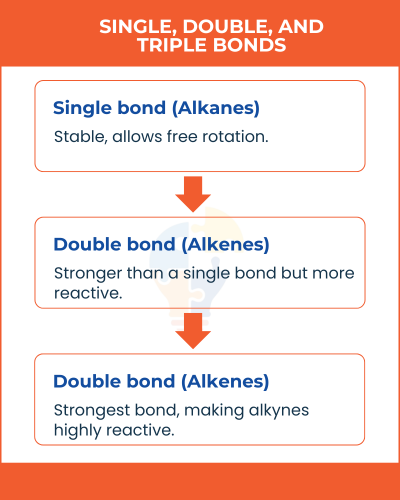
Single, Double, and Triple Bonds
- Single bond (Alkanes): Stable, allows free rotation.
- Double bond (Alkenes): Stronger than a single bond but more reactive.
- Triple bond (Alkynes): Strongest bond, making alkynes highly reactive.
The double or triple bond in unsaturated hydrocarbons makes them reactive in chemical reactions such as hydrogenation and polymerisation.
Now that you understand how structure affects reactivity, let’s explore the properties and uses of alkanes, alkenes, and alkynes in daily life.
Properties and Uses of Alkanes, Alkenes, and Alkynes
Alkanes, alkenes, and alkynes are key hydrocarbons in organic chemistry. Their bond types give them different physical and chemical properties.
These compounds play a big role in real life. We use them in fuels for cars, in plastics, and in many industries. Knowing their properties helps in choosing the right one for each use.
Now that you know their uses and importance, let’s look at a table that compares their chemical and physical properties clearly.
Physical Properties
| Property | Alkanes | Alkenes | Alkynes |
|---|---|---|---|
| Bond Type | Single bonds (saturated) | Double bonds (unsaturated) | Triple bonds (unsaturated) |
| Boiling Point | Increases with chain length | Lower than alkanes of similar size | Higher than alkenes and alkanes |
| Solubility | Insoluble in water but dissolves in organic solvents | Similar to alkanes | Similar to alkanes |
| State at Room Temp | Smaller alkanes are gases, larger ones are liquids or solids | Gases or liquids | Gases or liquids |
Chemical Properties
| Property | Alkanes | Alkenes | Alkynes |
|---|---|---|---|
| Combustion | Burns with a clean flame, producing CO₂ and H₂O | Burns with a smokier flame due to high carbon content | Produces high energy but burns with a sooty flame |
| Reactivity | Least reactive due to strong C-C single bonds | More reactive due to C=C double bonds | Most reactive due to C≡C triple bonds |
| Addition Reactions | Does not undergo addition reactions | Reacts with hydrogen, halogens, and water | Reacts with halogens and hydrogen faster than alkenes |
How Are Alkanes, Alkenes, and Alkynes Used in Daily Life?
Common Applications
| Use | Alkanes | Alkenes | Alkynes |
|---|---|---|---|
| Fuels | Petrol, diesel, natural gas | Used in high-energy fuels | Acetylene (ethyne) used in welding torches |
| Plastics | Used in paraffin wax and lubricants | Ethene used in making plastics (polyethene) | Not used in plastics |
| Pharmaceuticals | Used as solvents in medicines | Used in synthesis of drugs | Used in some organic reactions |
Industrial Significance
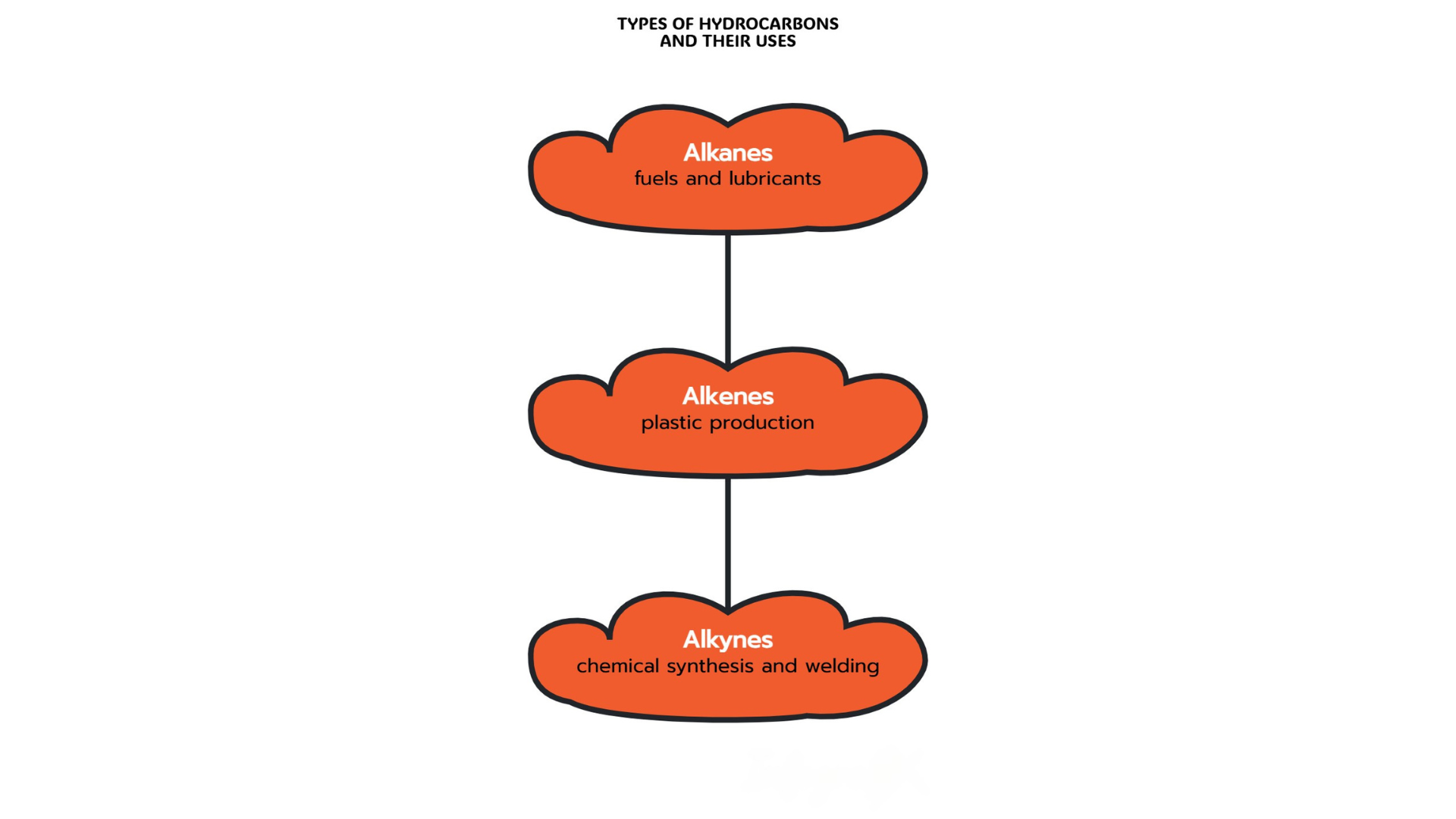
- Alkanes: Used as fuels and lubricants due to their stability.
- Alkenes: Important in plastic production, such as polyethene and polypropene.
- Alkynes: Used in chemical synthesis and high-temperature welding.
Why Are Alkanes Saturated and Alkenes/Alkynes Unsaturated?
Hydrocarbons, including alkanes, alkenes, and alkynes, are organic compounds made up of carbon and hydrogen atoms. They are classified based on the type of bonds between carbon atoms.
- Alkanes are saturated hydrocarbons because they contain only single bonds between carbon atoms.
- Alkenes and alkynes are unsaturated hydrocarbons because they contain at least one double or triple bond, respectively.
Understanding this difference helps explain their reactivity, chemical properties, and uses.
Saturation in Hydrocarbons
Alkanes: Saturated Hydrocarbons
Alkanes are considered saturated because they have only single bonds between carbon atoms. This means:

Example: Methane (CH₄) and Ethane (C₂H₆).
Alkenes and Alkynes: Unsaturated Hydrocarbons
- Alkenes contain at least one double bond between carbon atoms. Their general formula is CₙH₂ₙ.
- Alkynes contain at least one triple bond and have the general formula CₙH₂ₙ₋₂.
Because of their double or triple bonds, alkenes and alkynes are more reactive than alkanes and readily undergo addition reactions.
Example: Ethene (C₂H₄) and Ethyne (C₂H₂).
Impact on Chemical Behaviour
| Property | Alkanes (Saturated) | Alkenes & Alkynes (Unsaturated) |
|---|---|---|
| Bond Type | Single bonds | At least one double or triple bond |
| Reactivity | Low reactivity | More reactive due to double/triple bonds |
| Common Reactions | Substitution reactions | Addition reactions |
| Use in Industry | Fuels and lubricants | Plastics and chemical synthesis |
Since alkenes and alkynes have weaker π-bonds in their double and triple bonds, they are more likely to react than alkanes.
How Do Alkanes, Alkenes, and Alkynes React in Chemical Reactions?
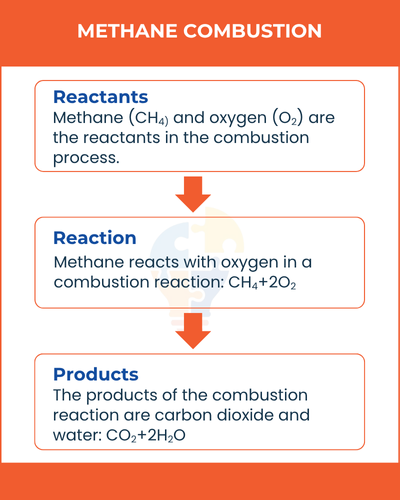
Combustion and Oxidation
All hydrocarbons undergo combustion when burned in oxygen, forming carbon dioxide and water.
Hydrocarbon + O2 → CO2 + H2O
- Complete combustion produces CO₂ and H₂O.
- Incomplete combustion (limited oxygen) produces carbon monoxide (CO) or carbon (soot).
Example:
Methane combustion: CH4+2O2→CO2+2H2O
Addition Reactions of Alkenes and Alkynes
Since alkenes and alkynes are unsaturated, they react easily with hydrogen, halogens, and water in addition reactions.
- Hydrogenation: Converts alkenes to alkanes by adding hydrogen.
C2H4+H2→C2H6
- Halogenation: Reaction with halogens (e.g., bromine water test for alkenes).
C2H4 + Br2 → C2H4Br2
Alkynes also undergo addition reactions, but in two steps, first forming an alkene, then an alkane.
Substitution Reactions in Alkanes
Since alkanes are saturated hydrocarbons, they do not readily undergo addition reactions. Instead, they react via substitution reactions, where one hydrogen atom is replaced by another functional group.
Example:
Methane reacts with chlorine under,
UV light:
CH4+Cl2→CH3Cl+HCl
This reaction continues, forming multiple substituted products like dichloromethane (CH₂Cl₂).
What Are the Differences Between Alkenes and Alkynes?
Alkenes and alkynes are both unsaturated hydrocarbons, meaning they contain carbon-carbon multiple bonds. However, they differ in structure, reactivity, and physical properties.
Structural Variations
- Alkenes contain at least one double bond between carbon atoms.
- Alkynes contain at least one triple bond between carbon atoms.
| Hydrocarbon Type | General Formula | Bond Type | Example |
|---|---|---|---|
| Alkene | CnH2n | One or more double bonds | Ethene (C2H4) |
| Alkyne | CnH2n−2 | One or more triple bonds | Ethyne (C2H2) |
Reactivity Differences
- Alkenes are more reactive than alkanes due to the presence of a double bond. They undergo addition reactions with hydrogen, halogens, and acids.
- Alkynes are even more reactive than alkenes due to the presence of a triple bond, making them highly reactive in addition reactions.
Example of Addition Reaction:
- Alkene Addition: Ethene reacts with bromine:
C2H4+Br2→C2H4Br2
- Alkyne Addition: Ethyne reacts with hydrogen:
C2H2+2H2→C2H6
How Are Alkanes, Alkenes, and Alkynes Named?
Naming hydrocarbons follows IUPAC naming rules, which standardise how organic molecules are named based on the number of carbon atoms and type of bonds.
IUPAC Naming Rules
- Find the longest carbon chain containing the functional group (double or triple bond).
- Number the carbon atoms to give the lowest number to the functional group.
- Identify and name side chains (if any).
- Use the correct suffix:
- “-ane” for alkanes (single bonds).
- “-ene” for alkenes (double bonds).
- “-yne” for alkynes (triple bonds).
Common Naming Conventions
| Hydrocarbon Type | Example | Molecular Formula |
|---|---|---|
| Alkane | Methane | CH4 |
| Alkene | Ethene | C2H4 |
| Alkyne | Ethyne | C2H2 |
How Can You Identify Alkanes, Alkenes, and Alkynes in a Lab?
Alkanes, alkenes, and alkynes are hydrocarbons with different chemical properties. Identifying them in a laboratory setting is crucial for understanding their reactivity and applications. Various chemical tests help distinguish these compounds, with the bromine water test being one of the most commonly used methods.
Bromine Water Test for Unsaturation
What Is the Bromine Water Test?
The bromine water test is used to identify whether a hydrocarbon is saturated or unsaturated. This test distinguishes alkanes (saturated hydrocarbons) from alkenes and alkynes (unsaturated hydrocarbons).
Procedure:
- Prepare the Test Tubes: Add 2 cm³ of bromine water to separate test tubes.
- Add the Hydrocarbon Sample: Introduce the alkane, alkene, or alkyne into the test tube.
- Observe the Colour Change: Shake the solution gently and note any changes.
"Get success in GCSE with our 1-on-1 GCSE chemistry tutor."
Results:
| Hydrocarbon Type | Reaction with Bromine Water | Explanation |
|---|---|---|
| Alkane | No colour change (remains orange-brown) | Alkanes contain only single bonds and do not react with bromine. |
| Alkene | Decolourises bromine water (turns colourless) | Alkenes contain a double bond, which reacts with bromine. |
| Alkyne | Decolourises bromine water (turns colourless) | Alkynes contain a triple bond, which also reacts with bromine. |
This reaction occurs because alkenes and alkynes undergo addition reactions, breaking the bromine molecule into two bromine atoms, which bond to the carbon-carbon double or triple bond.
Chemical Tests to Identify Alkanes, Alkenes, and Alkynes
1. Potassium Permanganate (KMnO₄) Test
This test, also called the Baeyer’s test, helps confirm the presence of an alkene or alkyne.
Procedure:
- Add a few drops of dilute potassium permanganate solution to the hydrocarbon sample.
- Shake the test tube and observe the colour change.
Results:
| Hydrocarbon Type | Reaction with KMnO₄ | Explanation |
|---|---|---|
| Alkane | No reaction, remains purple | Alkanes do not have double or triple bonds to react with KMnO₄. |
| Alkene | Turns brown, forming a precipitate | The double bond breaks, causing oxidation and forming a brown manganese dioxide precipitate. |
| Alkyne | Turns brown, forming a precipitate | The triple bond breaks, causing oxidation similar to alkenes. |
2. Combustion Test
Burning hydrocarbons in oxygen reveals differences in flame characteristics.
Results:
| Hydrocarbon Type | Flame Characteristics | Explanation |
|---|---|---|
| Alkane | Clean blue flame | Alkanes burn completely due to their saturated structure. |
| Alkene | Yellow smoky flame | Alkenes have higher carbon content, leading to incomplete combustion. |
| Alkyne | Very sooty flame | Alkynes have even higher carbon content, producing more soot. |
3. Reaction with Sulfuric Acid (H₂SO₄) Test
This test helps distinguish alkenes from alkanes.
Procedure:
- Add concentrated sulfuric acid to the hydrocarbon sample.
- Observe whether the solution forms a layer or dissolves.
Results:
| Hydrocarbon Type | Reaction with H₂SO₄ | Explanation |
|---|---|---|
| Alkane | No reaction, forms a separate layer | Alkanes do not react with sulfuric acid. |
| Alkene | Dissolves in sulfuric acid | Alkenes form an alkyl hydrogen sulfate compound. |
| Alkyne | Dissolves in sulfuric acid | Alkynes form a similar reactive intermediate. |
Read more Atomic Structure and Periodic table
FAQ’s
Why are alkenes more reactive than alkanes?
Alkenes contain a double bond, which creates a high electron density, making them more reactive than alkanes in chemical reactions.
Why are alkynes more reactive than alkanes and alkenes?
Alkynes contain a triple bond, which has higher electron density, making them more reactive in addition and substitution reactions.